AbstractUreolysis-driven microbially induced carbonate precipitation (MICP) is a naturally occurring process facilitated through microbial activities and biogeochemical reactions to produce calcium carbonate (CaCO3) mineral. MICP serves as an alternative ground improvement binder method to conventional technologies which is sustainable, requires low energy for its treatment process, results in a minimal carbon footprint and could offer economic benefits. In the last two decades, MICP has drawn great interest from the scientific community because of its practicality to stabilize granular soils, repair concrete cracks and remediate heavy metals. To obtain successful MICP application, it is vital to understand the conditions that favor its process. This paper, therefore, provides an overview of literature on CaCO3 precipitation mediated by ureolysis-driven MICP and its mechanism. The review includes a discussion on sources of urease enzyme from microorganisms used to induce CaCO3 crystal formation required for implementation of MCIP for ground improvement. Moreover, the key factors that influence the outcome of MICP and bio-engineering testing methods typically used to evaluate MICP performance are also highlighted. Finally, this review also provides insight on the current drawbacks (i.e. ammonium production, scale-up bioprocess and treatment cost) affecting MICP technology and recommendations for future consideration.
1. IntroductionCement is an important adhesion substance used in the construction industry for various applications (i.e. reservoirs, pavements, roads, tunnels, mortar and bricks). It is by mass the largest manufactured product on earth and the second most used substance in the world after water [1]. The global production of cement has increased tremendously since 1990 with an annual growth of 0.8–1.2%, and a predicted consumption rate of 3.7–4.4 billion tonnes by 2050 [2, 3]. In spite of the fact that cement utilization and other chemicals (asphalt) used as conventional construction materials and soil reinforcement, has led to urban development and global economic growth, its production has regrettably resulted in the release of large amounts of greenhouse gases such as carbon dioxide (CO2) into the environment [4]. This has shifted the need for more environmentally friendly building materials and manufacturing processes that will results in zero or minimal carbon footprint.
Microbially induced carbonate precipitation (MICP) is a biotechnological process which makes use of urease enzyme released from microorganisms and plants for biomineral precipitation under natural conditions. The inception of this technology dates back to the 1990s and has attracted stakeholders from various disciplines, particularly for its practicability as an innovative and effective approach for soil and ground improvement [5]. This review focuses on the ureolysis mechanism involving the calcium carbonate (CaCO3) bioprecipitation induced by microbial cells as an alternative to conventional engineering methods that can significantly improve soil engineering properties. A brief overview of conventional cement utilization for ground improvement and its ecological implications is presented. The emergence of ureolysis-driven MICP technology and urease sources are also discussed. The established conditions that influence MICP performance in soils are further presented. Aspects of quality control of ureolysis-driven MICP for industrial applications are discussed. Finally, current challenges affecting MICP and perspectives which provide future opportunities to advance this technology for real-world implementation are described.
2. Microbially Induced Carbonate PrecipitationLike other bio-mineralization process, MICP occurs under active biological settings and favourable environments to generate desired CaCO3 mineral formation. Currently, the acronym “MICP” is commonly used in various publications in the literature to describe “microorganisms induce calcium carbonate precipitation”, “microbiologically-induced calcite precipitation”, “microbiological carbonate precipitation”, microbially induced calcite precipitation” and “microbial carbonate precipitation”. However, early publications on this technology were referred to as “bacteriogenic precipitation of minerals”, “microbially mediated calcium carbonate precipitation”, “bacterially induced carbonate mineralization” and “microbiological precipitation of CaCO3”.
At present, there are six known MICP pathways (Table 1) that result in the saturation of CaCO3 crystals, namely, ureolysis (urea hydrolysis), photosynthesis, dissimilatory sulphate reduction, denitrification (nitrate reduction), ammonification of amino acids and methane oxidation. Readers are directed to a recent review by Castro-Alonso et al. [6] for more information. Of the six pathways, ureolysis-driven MICP is the least complex and most heavily researched method which has been subjected to immense biotechnological and engineering analyses. MICP is governed by the concentration of calcium ions, the concentration of dissolved inorganic carbon, pH, genetics of urease genes, CaCO3 polymorphisms and the availability of nucleation sites [7, 8]. These factors are essential for MICP pathways and influence the outcome of CaCO3 formation. MICP favours CaCO3 precipitation which primary binds soil particles and consequently leads to soil improvement (Fig. 1 and Fig. 2). Interesting, MICP has been successfully tested on various soil types (Table 2). The potential applications of MICP have been rigorously demonstrated under laboratory-scale and field-scale conditions to resolve several environmental and geotechnical engineering problems, i.e. soil stabilization, mitigating earthquake-induced soil liquefaction, erosion control, slope stabilization, bioremediation of heavy metal, wastewater treatment, concrete repair, soil liquefaction and enhanced oil recovery [9–15].
The importance of MICP technology for real-world application is reflected in the steady increase of research publications. An electronic bibliographical assessment of publications on MICP from 1999 to 2020 with title, author, and abstract were obtained from SCOPUS database. The search was carried out by using keywords “microbially induced carbonate precipitation”, “microbial urease” and “ureolytic”, which resulted in the retrieval of 410, 3041 and 475 publications, respectively. Fig. 3 showed there are few available publications in Scopus database with keywords “microbially induced carbonate precipitation”, and “ureolytic”. Nevertheless, Fig. 3 also indicate noteworthy increase in number of published papers since 2013. On the other hand, publications with the keyword “microbial urease” showed a steady increment, and saw an exponential growth in the recorded publication from double-to triple-digit numbers. Although microbial urease was not popularly used for research or industrial purposes until in recent decades, publications on urease enzyme derived from plants (jack bean and soybean) goes back to the 19th century.
3. Mechanism of Ureolysis-Driven MICPUreolysis-driven MICP utilizes microorganisms that secrete urease which catalyzes the hydrolysis of urea (Fig. 4). This hydrolysis subsequently results in equimolar amounts of ammonia and carbamate [Eq. (1)]. The hydrolysis of carbamate results in production of bicarbonate and ammonia [Eq. (2)]. According to Eqs. (3) and (4), these products subsequently equilibrate in the solution to form bicarbonate, ammonium and hydroxide, resulting in increased pH (alkaline) and the formation of carbonate ions [Eq. (5)]. Irrespective of the pH of the medium, urea hydrolysis leads to increased alkalinity, unless a buffer solution is added to help control the pH of the solution. At this point, soluble calcium ions in the solution are absorbed onto the bacterial cell surface because the bacterium is negatively charged [8]. This is also because the intracellular accumulation of calcium ions leads to excessive expulsion of protons, thus making the cells export calcium to compensate for the loss of protons [6]. Ureolysis then permits carbonate ions to bind with calcium ions and forms solid crystalline biomaterial (CaCO3) precipitates as shown in Eq. (6). The overall ureolysis-driven MICP reaction that demonstrates CaCO3 precipitation is shown in Eq. (7).
This then leads to supersaturation of carbonate precipitates induced on the surfaces of the cells. The mechanism of carbonate precipitation also reduces the high calcium ion concentrations. However, the chloride ions derived from CaCl2 may be toxic before the calcium ions have any impact on the cells. Hence, it is vital that appropriate concentrations of CaCl2 (if selected) are used to avoid killing the microbial cells prior to the production of the desired biominerals. More so, it is important to reiterate that for ureolysis-driven MICP, the bacterium plays two key roles: urease production which is needed to enforce urea hydrolysis and providing the nucleation site needed for CaCO3 precipitation [12].
The production of CaCO3 minerals induced by microorganisms has attracted numerous researchers globally for various potential applications [26]. CaCO3 is widely available as a natural inorganic compound (i.e. limestone and marble, coral, shellfish and snail shell). Its availability and accessibility have made it one of the most versatile materials known. Both naturally obtainable and precipitated CaCO3 are used globally for various industrial applications, e.g. sealant, pharmaceutical, food, paper and toiletries. The use of CaCO3 precipitated through MICP for soil stabilization has gained enormous interest among researchers. The progression of voided soil to cemented soil which results in stabilization and enhanced soil strength due to MICP (Fig. 5). The understanding of soil behaviour for over three centuries has focused on mechanical principles, geological processes and mineralogy, however, research in biotechnology and earth science has allowed crucial contributions of microorganism that mediate mineral formation and geochemical reactions [16].
4. Microbial Urease and Its SourcesHumans have long used microorganisms as sources of essential enzymes for numerous applications. These beneficial microorganisms are utilized in their natural forms or selectively bred to improve their performance. Microbial enzymes can be easily controlled physiologically and physio-chemically, are produced in large have quantities and can be inexpensively extracted using downstream processes [17, 18]. Besides, microorganisms can be readily manipulated to obtain relevant enzymes with desired characteristics [19]. The global market value for microbial enzymes was estimated at around US$ 4.2 billion in 2014 with a compound annual growth rate of approximately 7% and expected to reach nearly US$ 6.2 billion in 2020 [20]. It is suggested that the enzyme market for technical applications and process development will be more successful in the Asia-Pacific region and North America through 2021 [21]. Urease represents an historically important milestone because it was the first enzyme to be crystallized and shown to contain nickel [22]. Urease is capable of hydrolysing urea and is linked with protein degradation [23]. The scientific interest on microbial urease was largely due to its enzymatic activity because gastritis and stomach cancer are associated with infection from Helicobacter pylori [24]. Also, the role of microbial urease in enzymatic activity was primarily linked to the recycling of nitrogenous wastes and nitrogen assimilation [25]. In the search for bacterial species able to precipitate carbonate minerals, many investigators have focused on screening soils.
Microorganisms constitute between 70–85% of all living components within soil and it is estimated that a single kilogram of soil contains between 109 and 1012 microbes at the surface [16, 26]. Many researchers have reported the isolation of diverse microbial species from various soils capable of producing urease and CaCO3 precipitates (Table 3). Results from these investigations showed that the types of bacteria used for MICP experiments affects the enzyme activity, crystal formation and, ultimately, the overall outcome of the cementation process. S. pasteurii is a highly active ureolytic bacterium which has widely been studied for its potential applications in the construction industry. Although urease production occurs in numerous bacterial species, ureolytic activity is often associated with pathogenic bacteria [27]. S. pasteurii is non-pathogenic and is considered the most appropriate candidate for biomineralization activity due to its high urease production and versatility [28]. Indeed, enzyme production by S. pasteurii has been well documented in the literature [29–32] and this microbe is known for its ability to induce CaCO3 precipitates through MICP [33]. Based on the German Technical Rules for Biological Agents on the classification of prokaryotes (bacteria and archaea), S. pasteurii is a risk group 1 microorganism, making it unlikely to cause human disease [34]. This might be the reason most researchers prefer using S. pasteurii for construction and building purposes. Some of the earliest uses of S. pasteurii involved novel fluid permeability reduction and sand consolidation through precipitation of CaCO3 [8, 35].
5. Key Factors Influencing the Performance of MICPThe MICP requires biochemical and microbial activities in the physical environment to occur which can influence the outcome of this process. Successful MICP relies on sufficient urease production and CaCO3 precipitation, both of which can interchangeably affect soil cementation. For successful field-scale implementation, the aforementioned factors should be comprehensively studied and optimized. During field-scale trials, other factors will be unavoidable influences on MICP treatment [36]. Although there are several additional factors that can impact MICP (i.e. temperature, initial relative densities, curing duration, reaction time, varying soil particle sizes, organic contents, oil contaminants and freeze-thaw cycle, treatment cycle and flow, and anoxic condition), only the four major contributors are discussed in this review.
5.1. Bacterial Genotype and Cell ConcentrationThe key role of microorganisms has been attributed to their ability of producing urease and induce alkaline pH [37]. The bacterial cell surface is critical for CaCO3 precipitation because of the presence of negatively charged groups, thus allowing positively charged metal ions (i.e. Ca2+) to bind with the bacterial cell surface [38]. Of the many different microorganisms that have been widely reported to produce urease and cause CaCO3 precipitation, members of the genus Bacillus are the most common ureolytic bacteria isolated from local sources. However, S. pasteurii from the Sporosarcina group is the most used microorganism for multiple MICP applications. The selection of appropriate microorganisms for specific MICP applications is critical because different bacterial genotypes can result in diverse MICP outcomes. Some bacterial species may be able to generate greater biomass in a short period or require a less enriched nutrient medium for cultivation, however, they may not be suitable for MICP due to their toxicity, acidity and insufficient production of urease. The nature of exopolysaccharide secretion by different microorganism can also result in different MICP treatment process, thus it is essential to determine the appropriate ureolytic bacteria to use for various MICP applications. For example, Micrococcus yunnanensis, B. megaterium, Pararhodobactor sp., Lysinibacillus sphaericus and S. pasteurii are often used for the improvement of soil solidification, heavy metal remediation and crack repair especially in moderate to high temperate climate regions (30–60°C) [5, 31, 39–41]. Conversely, Lysinibacillus xylanilyticus has recently been reported to be a suitable MICP agent for soil improvement in cold regions (i.e. 15–25°C) [14, 42].
The concentration of bacterial cells used for MICP is another factor which needs consideration. The number of bacterial cells used per unit volume for MICP influences the level of biomineral formation. Ureolytic activity is dependent on the available substrate (e.g. urea) and the concentration of biomass cells, hence determining how the bacterial cell concentration influences urease production should be determined [36]. The cementation reagents and the bacterial cell concentration are two primary factors which control the degree of CaCO3 content homogeneity [43]. It has been suggested that higher bacterial cell concentrations (106–108 cells/mL) increases the amount of CaCO3 content during MICP [44]. This is because the cells are used to provide nucleation sites necessary for initiating the creation of an alkaline environment to induce growth of CaCO3 precipitates [7]. Okwadha and Li [44] showed that the increase in bacterial cells resulted in more CO32− production in the cementation solution, but this is not entirely supported by other studies. Zhao et al. [45] investigated how various cell concentrations influences soil biocementation. They cultivated S. pasteurii until it reached optical density (OD) of 0.3–1.5 and they observed that the urease activity, compressive strength, and CaCO3 content were all significantly influenced by an increase in bacterial cell concentration. In another study, which investigated the effect of bacterial cell concentration (OD 0.5–1.5) on the physico-mechanical property of cement mortar, it was reported that OD 1.0 resulted in the highest strength and lowest water absorption values when compared with other selected bacterial cell concentrations [46].
5.2. Cementation Reagents and Their ConcentrationsThe presence of cementation reagents such as urea and Ca2+ ions are essential for the ureolysis to occur. From the chemical perspective of MICP, the concentrations of carbonate and calcium sources are extremely important for CaCO3 precipitation. For this to occur, appropriate amounts of Ca2+ (supplemented externally) and urea (for CO32− production) are required. CaCl2 is commonly used as a precursor for producing CaCO3 crystals. However, unfortunately, chloride ions are regarded as harmful to building and construction materials because they can lead to corrosion or degradation of the pore structures. Chloride ion with concentrations below 0.4% by mass of cement have a low level of risk, while concentrations between 0.4% to 1.0% by mass of cement, and above 1% by mass of cement, represent medium and high levels of risk, respectively. Hence, the appropriate amount of CaCl2 should be used during MICP treatment, namely a molar ratio of 1.0 to 1.5, while for CaCO3 crystallization to occur during ureolysis, the molar ratio of ions to urea should range between 0.5 and 2.0 [34]. Some researchers have suggested the use of other calcium sources for MICP process which have different outcome due to the kinetics of biochemical reactions. Although, when compared with other calcium sources, it is widely suggested by researchers that calcium chloride is the most common used calcium for soil biocalcification [47]. The substitute calcium sources that are being used for CaCO3 formation are calcium acetate [Ca(C2H3O2)2], calcium oxide [CaO], calcium nitrate [Ca(NO3)2], calcium formate [Ca(HCOO)2], calcium lactate [C6H10CaO6] and calcium diglutamate [Ca(C5H8NO4)2] [47–52].
Several researchers have also investigated the use of alternative materials to replace analytical-grade cementation reagents (urea and CaCl2). Choi et al. [53] reported that eggshell mixed (ratio of 1:4) with diluted vinegar (5%, v/v) could be used to obtain soluble calcium and serve as an alternative to CaCl2 for soil biocementation. After treatment, biocemented soil samples were assessed at 335–392 kPa for compressive test, 1.62–6.54 × 10−6 m/s for permeability test and 4.4–8.2% for CaCO3 content. This research group later showed that calcium ions could also be obtained by mixing limestone from aggregate quarries with 7% (w/v) acetic acid derived from lignocellulosic biomass fast pyrolysis as a cost-effective alternative cementation reagent for MICP treatment [54]. Their MICP soil treatment resulted in CaCO3 content of 5.67–8.19%, while permeability was 8.17–1.52 × 10−6 m/s. Recently, Chen et al. [55] described the possibility of replacing synthetic urea with pig urine for CaCO3 precipitation. Their results suggested pig urine could permit CaCO3 crystals formation (43% more when compared with control sample) which allowed a decrease in permeability and posoity of the treated soil. The authors suggested that pig urine could serve as a cost-effective raw material for MICP, help reduce ammonia production and lower the carbon footprint.
5.3. Cultivation MediumComponents of the cultivation media may favour or inhibit essential biomass, enzymatic activities and biomineral precipitation, all of which are important features that make the MICP process more efficient. For most MICP studies, commercially procurable analytical-grade cultivation media, such as yeast extract, tryptic soy broth, nutrient broth and Luria (or lysogeny) broth, are commonly used for enrichment, culturing, enumeration and isolation of various microorganisms. Depending on the manufacturer, these culture media typically contain varying concentrations of their respective constituents to effectively support microbial growth. For example, nutrient broth contains yeast extract (1.5–5 g/L), peptone, (5–15 g/L), sodium chloride (5–6 g/L) and beef extract/glucose (1–3 g/L) at an initial pH of 7.4 ± 0.2 (at 25°C). Bacterial growth in most soils is often limited due to the lack of organic constituents. However, the constituents of cultivation media can affect the efficacy of MICP [55, 56]. De Muynck et al. [48] revealed that nutritional composition have a profound impact on the morphological formation of CaCO3 crystals. Their work indicated that when mortar specimens were treated with cementation solution containing nutrient broth, calcite rhombohedral crystals were absent but were abundant when cementation solution did not include nutrient broth. The presence of proteins in the nutrient broth can greatly influence the crystal growth pattern by adsorption of other proteins or organic matter [57, 58].
Williams et al. [32] studied the possibility of replacing yeast extract with other nutrient sources (lactose mother liquor, corn steep liquor, meat extract, glucose and sodium acetate) for MICP treatment of cement-based materials. Their investigation showed that a combination of urea/meat extract/sodium acetate served as the most suitable replacement for yeast extract to cultivate S. pasteurii. This alternative medium also allowed 75% retardation of cement hydration when compared with other nutrient sources and control sample (yeast extract). Recently, Kiasari et al. [59] explored the performance of different nutrient media to stimulate indigenous ureolytic bacteria for soil improvement. Six cultivation media, namely; glucose medium, yeast extract medium, sodium acetate medium, sugarcane molasses medium, Mol.2 YE.4 medium, Mol.4 YE.2 medium and reagent medium were selected. Results for shear strength (91.1–140%), compressive strength (430–450%) and CaCO3 content (6.8%–13.8%) showed that different growth medium constituents can affect MICP process. Their result also indicated that, despite the overall improved biocementation, samples which were subjected to yeast extract medium were most effective for stimulation of native ureolytic bacteria.
5.4. pHThe pH plays a vital role for bacterial transportation and adhesion to promote homogenous distribution of CaCO3 content and compressive strength of the treated soil. It is imperative to investigate how optimum pH conditions could be useful for obtaining better soil solidification process [122, 123]. Both acidic and basic conditions impact the outcome of MICP treatment which may result in lower compressive strength when compared to samples treated in neutral pH condition [62]. Given the importance of pH in influencing the MICP process, researchers now tend to determine the optimum pH conditions which will favour the performance of the selected bacterial species used in their experiments. Several investigations have indicated that pH 8–9 are the optimum condition for urease activity; these alkaline pH conditions are vital for ammonia production via ureolysis [30, 63]. Seifan et al. [64] investigated the effect of alkaline pH (9–12) on the production of CaCO3 and the bacterial cell concentration under controlled-pH batch conditions in 3 L laboratory-scale bioreactor at 35°C and 150 rpm for 180 h. Their work showed that the bacteria could grow in an alkaline pH environment, but the cell concentration decreased as the pH was increased. They also showed that cell viability reduced more than 2.5–fold at pH 10–12 when compared to pH 9.
Kim et al. [65] studied the optimal conditions of Staphylococcus saprophyticus and S. pasteurii for CaCO3 precipitation. Both microbes were injected into solution containing urea (1 g/L) and CaCl2 (14 g/L) with initial pH of the medium varying between 6 and 10. Their study indicated that when both ureolytic microorganisms were incubated at 30°C, alkaline pH produced the greater amount of CaCO3 precipitates. Their data showed that the precipitation difference in measured CaCO3 crystals at different initial medium pH was 25% and 60% for S. saprophyticus and S. pasteurii, respectively. Deng and Wang [66] tested the performance of S. pasteurii (ATCC 11859) during MICP treatment of coral sand. Their results showed that changes in different initial pH (8–11) did not significantly influence the outcome of MICP. The bacterial cell densities were all above OD of 1.0 after 24 h incubation at 30°C with shaking (180 rpm). This implied that different bacterial species would have different behaviour when cultivated in a media containing various pH levels. While pH may influence MICP, regulating the pH of cultivaiton media may not be a critical factor during field-scale experiment since ureolytic bacteria are capable of adapting to unfavourable pH environments. In a recent study performed by the current authors [67], large-scale bacterial cultivation was performed in a 3000 L custom-made reactor tank and the initial pH of the medium was not regulated. This did not affect the the growth performance of the bacteria, nor did it hinder the cells from producing sufficient urease and CaCO3 required for soil biocementation.
6. Testing Methods for Evaluation of MICP PerformanceIt is necessary to access and monitor the conditions of ureolysis-driven MICP for optimum performance and ensuring the products meet the required parameters. The evolution of MICP has been subjected to numerous analyses from multidisciplinary fields (Supplementary Materials, Fig. S1). Since ureolysis-driven MICP undergoes different biotechnological and engineering evaluations, several methods have been adopted over the last two decades to monitor its performance. These evaluations can also be adopted as standard processes for quality control and quality assurance of MICP during industrial implementation. There are numerous methods in the literature which are used to evaluate MICP performance, including scanning electron microscopy analysis, X-ray powder diffraction analysis, plasticity index characterization, cone penetration test, porosity and permeability tests. However, this review only discusses the commonly used methods to elucidate their efficiency in evaluating CaCO3 precipitation.
6.1. Urease ActivityQuantitative determination of bacterial urease activity is done using several analytical approaches. Urease activity through conductivity measurement is carried out after bacterial propagation [61]. A conductivity meter is used to determine the electrical changes after bacterial cultures are inoculated into a urea solution (1.5 M) and monitored for 25 ±2 °C [13]. High correlation coefficients usually indicate a positive linkage between the increase in conductivity and urea hydrolysis [13, 68]. Determination of urease activity by conductivity measurement is the most common method used in the literature, indicating it is a suitable indication for urease production [31, 45, 69, 70]. During the conductivity test, hydrolysis of urea leads to ammonium and carbonate ions production, thus leading to an increase in conductivity [69]. The conductivity variation rate is often acquired from the slope of the plotted graph with the inclusion of the dilution factor [31]. However, there are other notable methods which have been successfully used to determine urease production, including the phenol-hypochlorite assay, Nessler assay and colorimetric assay (Berthelot’s reaction) [7, 71–73]. These methods are suitable to study ammonia concentration notwithstanding the presence of calcium ions.
6.2. Biomass MeasurementThe viability of bacterial cells is greatly influenced by the conditions of their microenvironment. Monitoring cell growth provides essential information concerning the nutritional and proliferation conditions since different bacterial species behave differently during various cultivation conditions [74]. There are many classic and modern techniques (i.e. optical microscopy, colony counts, cell number estimation, turbidity measurement, dry weight determination) which are commonly used to determine cells biomass [74–76]. The OD or turbidimetry test is often used as a biomass concentration indicator to monitor the performance of MICP bacterial cells based on turbidity measurements [31]. Measuring the OD of growing cell cultures is a standard and simple microbiological method used to quantify important cultivation parameters (i.e. changes in bacterial morphology and biomass production) [77]. The number of bacterial cells is typically determined at a wavelength of 600 nm using an ultraviolet and visible spectrophotometer after zero point correction [12, 61]. The amount of absorbed light in a bacterial cell suspension can be immediately and directly related to bacterial mass or number [77]. Turbidimetry is the most widely utilized analytical tool for measurement of bacterial growth in liquid cultures due to its easy, fast and non-destructive feature [78]. Many MICP studies on ureolytic bacterial cultivation in various growth media (i.e. with or without urea) have successfully monitored the cell density or concentration using turbidity measurements [77, 79–81]. Moreover, the colony count method is also often used to monitor the growth behaviour of MICP microorganisms [82–84].
6.3. pH MeasurementThe influence of pH on MICP is indisputably important because it affects microbial activity, bacterial growth, urease activity, and CaCO3 precipitation [65]. Decomposition of urea by urease that releases NH3(g) and CO2(g) and the dissolution of NH3(g) in the urea-CaCl2 leads to a variation of pH during ureolysis process [85]. Hence, it is imperative to measure pH as a way of monitoring the performance of ureolytic bacteria during MICP experiments. Typically, a pH meter is used to measure the acidity or alkalinity of the bacterial culture or suspension [86]. The pH of ureolytic bacterial cells in the nutrient medium can be measured during MICP experiments at regular intervals for proper physiological characterization of their performance during cultivation [87]. The pH meter is usually calibrated with commercial pH standard before the pH measurement on samples is performed, after which the pH sensor is flushed with water before the pH of subsequent samples are determined [88]. MICP studies also involve the collection of effluents from various sampling sources or draining outlets during the MICP process for pH measurement [89–91]. Collected effluent samples are also subjected to cell viability or biomass and ammonium concentrations tests [92]. Changes in pH of effluent samples during or after MICP treatment provide a good indication of the state of the MICP process.
6.4. CaCO3 ContentThe CaCO3 content is one of the most critical outcomes of ureolysis-driven MICP. The amount of CaCO3 precipitated within the soil matrix has a substantial effect on the mechanical properties of the treated soil. Typically, CaCO3 content is often used to evaluate whether sand columns are well solidified by studying the distribution of CaCO3 content after curing is completed. Hence, measuring the CaCO3 content in soil specimens after MICP treatment is often performed. An in-vitro biomineralization test for CaCO3 content is often carried out in glassware (e.g. flask) to evaluate the CaCO3 capacities or urease activity of the selected MICP microorganisms [86, 93]. Flasks which contain a certain concentration of cementation fluid are inoculated with bacteria and incubated at a certain temperature. For evaluation of CaCO3 content from treated soil specimen which is often carried out after compressive strength test, the conventional gravimetric acid washing method is commonly used because of its easy operation and analysis [94, 95]. The difference between the two weights of the MICP-treated soil specimen is considered to be the weight of the precipitated carbonates [96]. Alternatively, ethylenediaminetetraacetic acid titration is used for determination of CaCO3 content from biocemented soil specimens [7, 97].
6.5. Compressive Strength TestThe compressive strength test is commonly performed to verify MICP on treated soil specimens under desired conditions [98]. The unconfirmed compressive strength (UCS) test is widely used in geotechnical engineering to measure the axial load of stress applied to the subjected tested specimen along a longitudinal axis. First, the consolidated granular soils are carefully dismantled from the columns before the UCS test is conducted under controlled conditions [99]. The UCS test is also frequently conducted to compare the performance of compressive strength of the treated soil samples for CaCO3 content [100]. However, in most cases, the soil specimen will be allowed to air-dry or cure (14 to 28 days) before disassembling the soil columns and subjecting the specimen to the UCS test. This test is often performed under laboratory conditions and the equipment is quite costly. However, there are other inexpensive ways to measure the strength of biocemented soils. Surface strength or local strength measurements are also used to obtain the desired compressive strength of consolidated soil specimens. This can be evaluated using soil pocket penetrometer, rebound hammer (Schmidt rebound hammer) or needle penetrometer tests.
7. Current Challenges and Perspectives on ureolysis-driven MICP7.1. Economic Feasibility of MICP TreatmentAs opposed to several soil improvement methods, MICP is still too expensive to be adopted for field-scale implementation. Until now, most MICP researchers use laboratory-grade growth media (e.g. nutrient broth and yeast extract) for biomass production which impedes MICP field-scale application. The MICP cost is also influenced by reagents required for soil treatments and specific treatment techniques used. There is currently limited information available in the literature on the cost of various MICP treatments. However, a few researchers have elaborated on MICP cost and its influence on field implementation. De Muynck et al. [101] indicated that the cost of MICP for surface treatments of sculptured and degraded stone ranged from US$29.71/m3 to US$51.67/m3. However, the cost of MICP treatment may be further reduced if efficient or optimized treatment techniques are implemented. MICP certainly has the potential to penetrate the commercial market because it can be used to produce construction materials using low temperature and renewable energy sources [102]. Even though the technology is expensive for scale-up and field-scale implementation, there are several companies like BioCement Technologies, Biomason and Bachy Solentanche that are currently using this technology for commercial engineering applications (i.e. bacteria-based additive to topsoil for prevention of erosion and bioconcrete production soil stabilization). For MICP to be accepted into the commercial market, it needs to show it is relatively inexpensive or similar in cost to existing alternatives technologies. For biocementation to be economically feasible, an important criteria need to be addressed, which is minimizing the costs of bacterial production cementation treatment [103]. Stimulating indigenous ureolytic microorganisms through injection methods may help eliminate the need for bacterial cultivation and reduce the treatment cost [104]. Interestingly, a recent review paper by Rahman et al. [105] presented a detailed cost assessment and environmental benefit of MICP technology using three scenarios: only paver blocks treated with MICP, pavers and sub-base layer treated with MICP, and pavers and subgrade treated with MICP. For further information, readers may refer to the publication [105] by these authors.
7.2. Ammonium ProductionA high concentration of ammonium is commonly produced during calcite precipitation, which has a detrimental impact to human health, soil, groundwater and the environment [106]. Ammonium levels in water of more than 0.5 mg/L can cause harm when consumed but this environmental issue remains largely ignored [107]. Recent investigations by Lee et al. [91, 108] on the removal of ammonium after MICP treatment showed that this problem can be resolved. They applied high pH (9–10) with a high ionic strength rinse solution (200–500 mM of CaCl2) to treat the effluent. Their treatment technique resulted in 99% removal of ammonium. Alternatively, Cheng et al. [108] recently showed that using a low pH (4.0) during MICP treatment resulted in more than 90% removal of ammonium. They also indicated that controlling the biomass concentration, urease activity and initial pH of the cementation solution resulted in a reduction of unwanted and harmful by-product (ammonia). However, this process may lower the precipitated CaCO3 minerals and result in weaker compressive strength. It will be interesting for future studies to investigate the effect of using this ammonia removal method on CaCO3 precipitation and overall MICP performance. For MICP to be regarded as a complete environmentally friendly technology, the dependence on urea for urease production need to be minimized. Urea is commonly used as a constituent of the cementation solution and growth media for the biocementation process and bacterial cell propagation, unfortunately, has a large greenhouse footprint. Most of the CO2 used to manufacture urea comes from CO2 generated during the production of ammonia, and is thus responsible for the high CO2 content of greenhouse gas emissions [109]. The global carbon footprint of technical-grade urea fertiliser ranges between 1.484 to 3.002 CO2eq/kg product (including CO2 captured in the product) [110]. Consequently, several researchers have recently investigated alternative sources to synthetic urea for MICP. Recent investigations on human and pig urine samples serving as an alternative source of urea for MICP applications were reported [55, 112]. These studies were able to successfully substitute analytical-grade and industrial-grade urea with urine and produce biocement bricks/columns. Their findings also show that MICP cannot only become a green and sustainable technology, it can also be used for recycling of waste materials and mitigate environmental pollution. Future research can also consider other alternatives as sources of urea for MICP experiments. Nitrogen sources which may be investigated as a replacement to urea for MICP process are inorganic fertilizers (i.e. ammonium sulphate, calcium ammonium nitrate, urea-ammonium sulphate, liquid urea-ammonium nitrate and environmentally smart nitrogen) and organic fertilizers (i.e. animal manure and slurry, industrial wastewater and sewage).
7.3. Large-scale Production of Ureolytic BacteriaGrowing large-scale volumes of ureolytic bacterial cultures with standard laboratory growth medium and commercial bioreactors makes MICP too costly. Also, most conventional MICP applications are performed using sterile cultivation medium and processes to grow the bacterial cells, but in real-world situations, it is not necessary to use sterile conditions because this adds to the bacterial production costs. Hence, in certain situations where field-scale investigation will be carried out, it is not necessary to use non-sterile growth condition for cultivation of ureolytic bacteria since the cells will out-compete other non-desired microorganisms when urea is added or ammonia production occurs [112]. Future studies can adopt the use of a single custom-built reactor for economic scale-up of bacterial production. It would be essential to also have systems attached to the custom-built reactor that can minimize contamination. Also, in a situation where a high concentration of urea is needed during bacterial cultivation (which also helps to reduce non-beneficial microbes), outlet pipes can be installed in the reactor that allows collection of ammonia gas produced during the ureolysis process. These pipes can be connected to a simple custom-built device that acts as a wet scrubber for removal of pollutant gas. One of the earliest studies on non-sterile production of ureolytic bacteria for the scale-up purpose was carried out by Whiffin [13]. The author showed that a custom-built fibreglass airlift pilot-scale reactor (120 L capacity) was able to support the growth of S. pasteurii at 30°C under non-sterile conditions when placed on-site. Industrial-grade Vegemite acetate medium (13.5 g/L), urea (10 g/L1) with an initial pH of 8.15 were used for the pilot-scale bacterial cultivation. Although lower urease activity and biomass production were obtained after cultivation, successful biocementation of the treated soil occurred. Aoki et al. [113] recently reported using a low-technology down-flow hanging sponge reactor (170 cm3 capacity) to cultivate ureolytic bacteria from samples collected from a reservoir tank. The non-sterile enrichment cultivation (130 days) occurred at 25°C using yeast extract-based medium which contained 0.17 M urea. Their results showed high urease activity (10 μmoL urea hydrolyzed/min/mL) and CaCO3 precipitates (92 ± 7 mg/mL). Very recently, Omoregie et al. [67] demonstrated that a custom-built stainless steel reactor (3 m3) could be used to sequentially scale-up the production of bacterial cultures under non-sterile conditions and with low-cost cultivation medium for in-situ ureolysis-driven MICP application. At the end of the 90 h cultivation under non-controlled conditions, they reported an OD of 2 and urease activity of 11 mM urea hydrolysed/min.
8. ConclusionsUreolysis-driven MICP is a biomineralization process used to induce CaCO3 precipitates for various engineering applications. This paper reviewed the scientific literature on ureolysis-driven MICP to validate the conditions that efficiently influences its mechanism. In addition, this review also discussed the common methods used to evaluate MICP performance. These methods can be adopted as quality control protocols for industrial applications. MICP may be in its infancy, but rigorous analyses and evidences gathered from the literature has shown that growing trend on the need to develop this technology for field-scale or in-situ applications. While existing commercial companies that use ureolysis-driven MICP techniques are few, the growing demand for more innovative and environmentally friendly soil binder technologies will lead to increased commercial interest. For the past two decades, most investigations focused on laboratory-scale works such as ureolytic bacterial identification and selection, evaluating the factors that influence bacterial cell concentration, pH and urease activity on biomineralization, and treatment techniques on granular soil. Based on the literature reviewed, these studies have improved the knowledge available on the MICP process. However, more emphasis has to be given to resolving the existing challenges affecting this technology especially high operating costs of MICP treatment, formation of undesirable by-products (ammonium) and large-scale production of biomass for field-scale application. The key factors (i.e. pH, cultivation medium, bacterial cell concentration and cementation reagents) affecting MICP performance presented in this review will encourage future MICP researchers to use this knowledge to critically investigate how they can maintain optimum performance of ureolysis-driven MICP and effectively resolve the existing problems affecting adoption of this technology. If future developments can successfully avoid or manage ammonium pollution and reduce high operating costs, this will help promote the industrial application of ureolysis-driven MICP technology.
AcknowledgementsAO acknowledges the School of Research Office (Swinburne University of Technology Sarawak Campus, Sarawak, Malaysia) for the studentship given to cover his PhD academic study. AO is also thankful to Dr Ngu Lock Hei (Associate Dean, Curriculum Enhancement and Accreditation, Swinburne University of Technology Sarawak campus, Malaysia), Prof Clem Kuek (Adjunct Visiting Researcher, Curtin University, Australia) and Dr Dominic Ek Leong Ong (Senior Lecturer, School of Engineering and Built Environment, Griffith University, Australia) for their respective opinions used for discussion in this review paper on quality control and quality assurance of MICP for industrial application.
References1. Scrivener KL, John VM, Gartner EM. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem Concr Res. 2018;114:2–26.
2. Benhelal E, Zahedi G, Shamsaei E, Bahadori A. Global strategies and potentials to curb CO2 emissions in cement industry. J Clean Prod. 2013;51:142–161.
3. Andrew RM. Global CO2 emissions from cement production, 1928–2017. Earth Syst Sci Data. 2018;10:2213–2239.
4. Imbabi MS, Carrigan C, McKenna S. Trends and developments in green cement and concrete technology. Int J Sustain Built Environ. 2012. 1:194–216.
5. Al Imran M, Gowthaman S, Nakashima K, Kawasaki S. The influence of the addition of plant-based natural fibers (jute) on biocemented sand using MICP method. Materials. 2020;13:4198.
6. Castro-Alonso MJ, Montañez-Hernandez LE, Sanchez-Muñoz MA, Macias Franco MR, Narayanasamy R, Balagurusamy N. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front Mater. 2019;6:126–140.
7. Stocks-Fischer S, Galinat JK, Bang SS. Microbiological precipitation of CaCO3
. Soil Biol Biochem. 1999;31:1563–1571.
8. Ferris FG, Stehmeier LG, Kantzas A, Mourits FM. Bacteriogenic mineral plugging. J Can Pet Technol. 1997;35:56–61.
9. Kalhori H, Bagherpour R. Application of carbonate precipitating bacteria for improving properties and repairing cracks of shotcrete. Constr Build Mater. 2017;148:249–260.
10. Lin H, Suleiman MT, Jabbour HM, Brown DG, Kavazanjian E. Enhancing the axial compression response of pervious concrete ground improvement piles using biogrouting. Geotech Geoenviron Eng. 2016;142:04016045–04016056.
11. Wu J, Wang X-B, Wang H-F, Zeng RJ. Microbially induced calcium carbonate precipitation driven by ureolysis to enhance oil recovery. RSC Adv. 2017;7:37382–37391.
12. Tian K, Wu Y, Zhang H, Li D, Nie K, Zhang S. Increasing wind erosion resistance of aeolian sandy soil by microbially induced calcium carbonate precipitation. L Degrad Dev. 2018;29:4271–4281.
13. Whiffin VS. Microbial CaCO3 precipitation for the production of biocement [dissertation]. Perth: Murdoch Uni; 2004.
14. Gowthaman S, Iki T, Nakashima K, Ebina K, Kawasaki S. Feasibility study for slope soil stabilization by microbial induced carbonate precipitation (MICP) using indigenous bacteria isolated from cold subarctic region. SN Appl Sci. 2019;1:1480–1495.
15. Li M, Cheng X, Guo H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int Biodeterior Biodegrad. 2013;76:81–85.
16. Mitchell JK, Santamarina JC. Biological Considerations in Geotechnical Engineering. J Geotech Geoenviron Eng. 2005;131:1222–1233.
17. Ibrahim CO. Development of applications of industrial enzymes from Malaysian indigenous microbial sources. Bioresour Technol. 2008;99:4572–4582.
18. Binod P, Palkhiwala P, Gaikaiwari R, Nampoothiri KM, Duggal A, Dey K, Pandey A. Industrial enzymes - Present status and future perspectives for India. J Sci Ind Res. 2013;72:271–86.
19. Rezaul Kar KM, Husaini A, Tasnim T. Production and characterization of crude glucoamylase from newly isolated Aspergillus flavus NSH9 in liquid culture. Am J Biochem Mol Biol. 2017;7:118–126.
20. Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:174–189.
21. Chapman J, Ismail AE, Dinu CZ. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts. 2018;8:238–264.
22. Weeks DL, Eskandari S, Scott DR, Sachs G. A H+-Gated urea channel: The link between helicobacter pylori urease and gastric colonization. Science. 2000;287:482–485.
23. Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev. 1995;59:451–480.
24. Mobley HLT, Garner RM, Bauerfeind P.
Helicobacter pylori nickel-transport gene nixA: synthesis of catalytically active urease in Escherichia coli independent of growth conditions. Mol Microbiol. 1995;16:97–109.
25. Mora D, Arioli S. Microbial urease in health and disease. PLoS Pathog. 2014;10:10–13.
26. Benini S, Rypniewski WR, Wilson KS, Miletti S, Ciurli S, Mangani S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis costs two nickels. Structure. 1999;7:205–216.
27. Konieczna I, Zarnowiec P, Kwinkowski M, Kolesinska B, Fraczyk J, Kaminski Z, et al. Bacterial urease and its role in long-lasting human diseases. Curr Protein Pept Sci. 2013;13:789–806.
28. Oualha M, Bibi S, Sulaiman M, Zouari N. Microbially induced calcite precipitation in calcareous soils by endogenous Bacillus cereus, at high pH and harsh weather. J Environ Manage. 2020;257:109965.
29. Van Der Star WRL, Taher E, Harkes MP, Blauw M, Van Loosdrecht MCM, Van Paassen LA. Use of waste streams and microbes for in situ transformation of sand into sandstone. Gr Improv Technol Case Hist. 2009;177–82.
30. van Paassen L. Biogrout: Ground Improvement by Microbially Induced Carbonate Precipitation [dissertation]. Delft: Delft Uni. of Tech; 2009.
31. Omoregie AI, Khoshdelnezamiha G, Senian N, Ong DEL, Nissom PM. Experimental optimisation of various cultural conditions on urease activity for isolated Sporosarcina pasteurii strains and evaluation of their biocement potentials. Ecol Eng. 2017;109:65–75.
32. Williams SL, Kirisits MJ, Ferron RD. Optimization of growth medium for Sporosarcina pasteurii in bio-based cement pastes to mitigate delay in hydration kinetics. J Ind Microbiol Biotechnol. 2016;43:567–75.
33. Ghosh T, Bhaduri S, Montemagno C, Kumar A.
Sporosarcina pasteurii can form nanoscale calcium carbonate crystals on cell surface. PLoS One. 2019;14:e0210339.
34. Ivanov V, Stabnikov V, Stabnikova O, Kawasaki S. Environmental safety and biosafety in construction biotechnology. World J Microbiol Biotechnol. 2019;35:26.
35. Kantzas A, Stehmeier L, Marentette DF, Ferris FG, Jha KN, Maurits FM. A novel method of sand consolidation through bacteriogenic mineral plugging. In : Annual Technical Meeting; 7–10 June 1992; Calgary, Alberta. p. 46–62.
36. Mortensen BM, Haber MJ, Dejong JT, Caslake LF, Nelson DC. Effects of environmental factors on microbial induced calcium carbonate precipitation. J Appl Microbiol. 2011;111:338–349.
37. Mitchell AC, Ferris FG. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim Cosmochim Acta. 2005;69:4199–4210.
38. Rivadeneyra MA, Delgado G, Ramos-Cormenzana A, Delgado R. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: Crystal formation sequence. Res Microbiol. 1998;149:277–287.
39. Fujita M, Nakashima K, Achal V, Kawasaki S. Whole-cell evaluation of urease activity of Pararhodobacter sp. isolated from peripheral beachrock. Biochem Eng J. 2017;124:1–5.
40. Soon NW, Lee LM, Khun TC, Ling HS. Factors affecting improvement in engineering properties of residual soil through microbial induced calcite precipitation. J Geotech Geoenviron Eng. 2014;140:04014006–04014016.
41. Achal V, Pan X, Zhang D, Fu Q. Bioremediation of Pb-contaminated soil based on microbially induced calcite precipitation. J Microbiol Biotechnol. 2012;22:244–247.
42. Gowthaman S, Nakashima K, Kawasaki S. Freeze-thaw durability and shear responses of cemented slope soil treated by microbial induced carbonate precipitation. Soils Found. 2020;60:840–855.
43. Nayanthara PGN, Dassanayake ABN, Nakashima K, Kawasaki S. Microbial induced carbonate precipitation using a native inland bacterium for beach sand stabilization in nearshore areas. Appl Sci. 2019;9:3201–2123.
44. Okwadha GDO, Li J. Optimum conditions for microbial carbonate precipitation. Chemosphere. 2010;89:1143–1148.
45. Zhao Q, Li L, Li C, Li M, Amini F, Zhang H. Factors affecting improvement of engineering properties of micp-treated soil catalyzed by bacteria and urease. J Mater Civ Eng. 2014;26:04014094–04014103.
46. Abo-El-Enein SA, Ali AH, Talkhan FN, Abdel-Gawwad HA. Application of microbial biocementation to improve the physico-mechanical properties of cement mortar. HBRC J. 2013;9:36–40.
47. Tang Y, Lian J, Xu G, Yan Y, Xu H. Effect of cementation on calcium carbonate precipitation of loose sand resulting from microbial treatment. Trans Tianjin Univ. 2017;23:547–554.
48. De Muynck W, Cox K, DeBelie N, Verstraete W. Bacterial carbonate precipitation as an alternative surface treatment for concrete. Constr Build Mater. 2008;22:875–885.
49. Fahmi A, Katebi H, Hajialilue Bonab M, Samadi Kafil H. Microbial sand stabilization using corn steep liquor culture media and industrial calcium reagents in cementation solutions. Ind Biotechnol. 2018;14:270–275.
50. Tayebani B, Mostofinejad D. Self-healing bacterial mortar with improved chloride permeability and electrical resistance. Constr Build Mater. 2019;208:75–86.
51. Jing X, Wu Y. Multiscale mechanical quantification of self-healing concrete incorporating non-ureolytic bacteria-based healing agent. Cem Concr Res. 2014;64:1–10.
52. Ronholm J, Schumann D, Sapers HM, Izawa M, Applin D, Berg B, Mann P, Vali H, Flemming RL, Cloutis EA, Whyte LG. A mineralogical characterization of biogenic calcium carbonates precipitated by heterotrophic bacteria isolated from cryophilic polar regions. Geobiology. 2014;12:542–556.
53. Choi S-G, Wu S, Chu J. Biocementation for sand using an eggshell as calcium source. J Geotech Geoenviron Eng. 2016;142:6016010–6016013.
54. Choi SG, Chu J, Brown RC, Wang K, Wen Z. Sustainable biocement production via microbially induced calcium carbonate precipitation: Use of limestone and acetic acid derived from pyrolysis of lignocellulosic biomass. ACS Sustain Chem Eng. 2017;5:5183–5190.
55. Chen H-J, Huang Y-H, Chen C-C, Maity JP, Chen C-Y. Microbial induced calcium carbonate precipitation (MICP) using pig urine as an alternative to industrial urea. Waste Biomass Valori. 2018;10:2887–2895.
56. Khan YM, Munir H, Anwar Z. Optimization of process variables for enhanced production of urease by indigenous Aspergillus niger strains through response surface methodology. Biocatal Agric Biotechnol. 2019;20:101202.
57. Al-Salloum Y, Abbas H, Sheikh QI, Hadi S, Alsayed S, Almusallam T. Effect of some biotic factors on microbially-induced calcite precipitation in cement mortar. Saudi J Biol Sci. 2017;24:286–294.
58. Hammes F, Boon N, De Villiers J, Verstraete W, Siciliano SD, Villiers JD. Strain-specific ureolytic microbial calcium carbonate precipitation. Appl Environ Microbiol. 2003;69:4901–4909.
59. Amini Kiasari M, Pakbaz MS, Ghezelbash GR. Comparison of effects of different nutrients on stimulating indigenous soil bacteria for biocementation. J Mater Civ Eng. 2019;31:04019067–04019079.
60. Imran MA, Kimura S, Nakashima K, Evelpidou N, Kawasaki S. Feasibility study of native ureolytic bacteria for biocementation towards coastal erosion protection by MICP method. Appl Sci. 2019;9:4462.
61. Harkes MP, van Paassen LA, Booster JL, Whiffin VS, van Loosdrecht MCM. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol Eng. 2010;36:112–117.
62. Mujah D, Shahin M, Cheng L. Performance of biocemented sand under various environmental conditions. In : Proceedings of the XVIII Brazilian conference on soil mechanics and geotechnical engineering; 19–22 October 2016; Belo Horizonte, Minas Gerais. p. 1–8.
63. Stabnikov V, Jian C, Ivanov V, Li Y. Halotolerant, alkaliphilic urease-producing bacteria from different climate zones and their application for biocementation of sand. World J Microbiol Biotechnol. 2013;29:1453–1460.
64. Seifan M, Samani AK, Berenjian A. New insights into the role of pH and aeration in the bacterial production of calcium carbonate (CaCO3). Appl Microbiol Biotechnol. 2017;101:3131–3142.
65. Kim G, Kim J, Youn H. Effect of temperature, pH, and reaction duration on microbially induced calcite precipitation. Appl Sci. 2018;8:1277–1286.
66. Deng W, Wang Y. Investigating the factors affecting the properties of coral sand treated with microbially induced calcite precipitation. Adv Civ Eng. 2018;8:1–6.
67. Omoregie AI, Palombo EA, Ong DEL, Nissom PM. A feasible scale-up production of Sporosarcina pasteurii using custom-built stirred tank reactor for in-situ soil biocementation. Biocatal Agric Biotechnol. 2020;24:101544.
68. Al-Thawadi SM. High strength in-situ biocementation of soil by calcite precipitating locally isolated ureolytic bacteria. [dissertation]. Perth: Murdoch Uni; 2008.
69. Sun X, Miao L, Tong T, Wang C. Study of the effect of temperature on microbially induced carbonate precipitation. Acta Geotech. 2019;14:627–638.
70. Wang T, Wang S, Tang X, Fan X, Yang S, Yao L, Li Y, Han H. Isolation of urease-producing bacteria and their effects on reducing Cd and Pb accumulation in lettuce (Lactuca sativa L.). Environ Sci Pollut Res. 2020;27:8707–8718.
71. Dhami NK, Quirin MEC, Mukherjee A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves. Ecol Eng. 2017;103:106–117.
72. Chen X, Achal V. Biostimulation of carbonate precipitation process in soil for copper immobilization. J Hazard Mater. 2019;368:705–713.
73. Cheng L, Shahin MA, Cord-Ruwisch R. Bio-cementation of sandy soil using microbially induced carbonate precipitation for marine environments. Géotechnique. 2014;64:1010–1013.
74. Koch AL. Growth measurement. Methods for General and Molecular Microbiology. 3rdAmerican Society of Microbiology; 2007. p. 172–199.
75. Holm-Hansen O, Karl DM. Biomass and adenylate energy charge determination in microbial cell extracts and environmental samples. Methods Enzymol. 1978;57:73–85.
76. Pringle JR, Mor JR. Methods for monitoring the growth of yeast cultures and for dealing with the clumping problem. Methods Cell Biol. 1975;11:131–168.
77. Kim J-H, Lee J-Y. An optimum condition of MICP indigenous bacteria with contaminated wastes of heavy metal. J Mater Cycles Waste Manag. 2019;21:239–247.
78. Maia MRG, Marques S, Cabrita ARJ, Wallace RJ, Thompson G, Fonseca AJ, Oliveira HM. Simple and versatile turbidimetric monitoring of bacterial growth in liquid cultures using a customized 3D printed culture tube holder and a miniaturized spectrophotometer: Application to facultative and strictly anaerobic bacteria. Front Microbiol. 2016;7:1381–1393.
79. Phang IR, San Chan Y, Wong KS, Lau SY. Isolation and characterization of urease-producing bacteria from tropical peat. Biocatal Agric Biotechnol. 2018;13:168–175.
80. Wu M, Hu X, Zhang Q, Xue D, Zhao Y. Growth environment optimization for inducing bacterial mineralization and its application in concrete healing. Constr Build Mater. 2019;209:631–643.
81. Sun X, Miao L, Tong T, Wang C. Improvement of microbial-induced calcium carbonate precipitation technology for sand solidification. J Mater Civ Eng. 2018;30:4018301.
82. Arias D, Cisternas LA, Rivas M. Biomineralization of calcium and magnesium crystals from seawater by halotolerant bacteria isolated from Atacama Salar (Chile). Desalination. 2017;405:1–9.
83. Han Z, Li D, Zhao H, Yan H, Li P. Precipitation of carbonate minerals induced by the halophilic Chromohalobacter Israelensis under high salt concentrations: Implications for natural environments. Minerals. 2017;7:95–121.
84. Grabiec AM, Starzyk J, Stefaniak K, Wierzbicki J, Zawal D. On possibility of improvement of compacted silty soils using biodeposition method. Constr Build Mater. 2017;138:134–140.
85. Li W, Chen WS, Zhou PP, Cao L, Yu LJ. Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase. Colloids Surf B:Biointerfaces. 2013;102:281–287.
86. Omoregie AI, Ngu LH, Ong DEL, Nissom PM. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal Agric Biotechnol. 201917:247–255.
87. Achal V, Pan X. Influence of calcium sources on microbially induced calcium carbonate precipitation by Bacillus sp. CR2. Appl Biochem Biotechnol. 2014;173:307–317.
88. Soon NW. Improvements in engineering properties of tropical residual soil by microbially-induced calcite precipitation [dissertation]. Perak: Uni Tunku Abdul Rahman; 2013.
89. Lee LM, Ng WS, Tan CK, Hii SL. Bio-mediated soil improvement under various concentrations of cementation reagent. Appl Mech Mater. 2012;204–208:326–329.
90. Omoregie AI, Palombo EA, Ong DEL, Nissom PM. Biocementation of sand by Sporosarcina pasteurii strain and technical-grade cementation reagents through surface percolation treatment method. Constr Build Mater. 2019;228:116828.
91. Lee M, Gomez MG, San Pablo ACM, Kolbus CM, Graddy CM, DeJong JT, Nelson DC. Investigating ammonium by-product removal for ureolytic bio-cementation using meter-scale experiments. Sci Rep. 2019;9:1–5.
92. Cheng L, Cord-Ruwisch R. Selective enrichment and production of highly urease active bacteria by non-sterile (open) chemostat culture. J Ind Microbiol Biotechnol. 2013;40:1095–1104.
93. Dhami NK, Alsubhi WR, Watkin E, Mukherjee A. Bacterial community dynamics and biocement formation during stimulation and augmentation: Implications for soil consolidation. Front Microbiol. 2017;8:1267–1283.
94. Choi S-G, Park S-S, Wu S, Chu J. Methods for calcium carbonate content measurement of biocemented soils. J Mater Civ Eng. 2017;29:6017015–6017018.
95. Mahawish A, Bouazza A, Gates WP. Unconfined compressive strength and visualization of the microstructure of coarse sand subjected to different biocementation levels. J Geotech Geoenvironm Eng. 2019;145:4019033.
96. Rebata-Landa V. Microbial activity in sediments: effects on soil behavior [dissertation]. Georgia:Georgia Inst of Tech. 2007.
97. Chu J, Stabnikov V, Ivanov V. Microbially Induced calcium carbonate precipitation on surface or in the bulk of soil. Geomicrobiol J. 2012;29:544–549.
98. Cheng L, Cord-Ruwisch R, Shahin MA. Cementation of sand soil by microbially induced calcite precipitation at various degrees of saturation. Can Geotech J. 2013;50:81–90.
99. Wen K, Li Y, Liu S, Bu C, Li L. Development of an improved immersing method to enhance microbial induced calcite precipitation treated sandy soil through multiple treatments in low cementation media concentration. Geotech Geol Eng. 2019;37:015–1027.
100. Cheng L, Shahin MA, Cord-Ruwisch R, Addis M, Hartanto T, Elms C. Soil Stabilisation by microbial-induced calcite precipitation (MICP): Investigation into some physical and environmental aspects. In : Abdelmalek B, Brown B, Yuen S, editors7th International Congress on Environmental Geotechnics; Melbourne, Australia. 2014; p. 10–14.
101. De Muynck W, Verbeken K, De Belie N, Verstraete W. Influence of urea and calcium dosage on the effectiveness of bacterially induced carbonate precipitation on limestone. Ecol Eng. 2010;36:99–111.
102. Myhr A, Røyne F, Brandtsegg AS, et al. Towards a low CO2 emission building material employing bacterial metabolism (2/2): Prospects for global warming potential reduction in the concrete industry. PLoS One. 2019;14:e0208643.
103. Kakelar MM, Ebrahimi S, Hosseini M. Improvement in soil grouting by biocementation through injection method. Asia-Pacific J Chem Eng. 2016;11:930–938.
104. Gomez MG, Graddy CMR, DeJong JT, Nelson DC. Biogeochemical changes during bio-cementation mediated by stimulated and augmented ureolytic microorganisms. Sci Rep. 2019;9:11517–11531.
105. Rahman MM, Hora RN, Ahenkorah I, Beecham S, Karim MR, Iqbal A. State-of-the-art review of microbial-induced calcite precipitation and its sustainability in engineering applications. Sustainability. 2020;12:6281.
106. van Paassen LA, Ghose R, van der Linden TJM, van der Star WR, van Loosdrecht MC. Quantifying biomediated ground improvement by ureolysis: large-scale biogrout experiment. J Geotech Geoenvironl Eng. 2010;136:1721–1728.
107. Lee M, Kolbus CM, Yepez AD, Gomez MG. Investigating ammonium by-product removal following stimulated ureolytic microbially-induced calcite precipitation. In : Geo-Congress 2019: Soil Improvement; Reston, VA: American Society of Civil Engineers; 2019. p. 260–272.
108. Cheng L, Shahin MA, Chu J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019;14:615–626.
109. Shi L, Liu L, Yang B, Sheng G, Xu T. Evaluation of industrial urea energy consumption (EC) based on life cycle assessment (LCA). Sustainability. 2020;12:3793.
110. Antione H, Bjarne C. The carbon footprint of fertiliser production: regional reference values, the International Fertiliser Society. Prague, Czech Republic: International Fertiliser Society; 2019. p. 1–21.
111. Lambert SE, Randall DG. Manufacturing bio-bricks using microbial induced calcium carbonate precipitation and human urine. Water Res. 2019;160:158–166.
112. Graddy CMR, Gomez MG, Kline LM, Morrill SR, DeJong JT, Nelson DC. Diversity of Sporosarcina-like bacterial strains obtained from meter-scale augmented and stimulated biocementation experiments. Environ Sci Technol. 2018;52:997–4005.
113. Aoki M, Noma T, Yonemitsu H, Araki N, Yamaguchi T, Hayashi K. A low-tech bioreactor system for the enrichment and production of ureolytic microbes. Polish J Microbiol. 2018;67:59–65.
114. Liu D, Shao A, Li H, Jin C, Li Y. A study on the enhancement of the mechanical properties of weak structural planes based on microbiologically induced calcium carbonate precipitation. Bull Eng Geol Environ. 2020;79:4349–4362.
115. Naveed M, Duan J, Uddin S, Suleman M, Hui Y, Li H. Application of microbially induced calcium carbonate precipitation with urea hydrolysis to improve the mechanical properties of soil. Ecol Eng. 2020;153:105885.
116. Sidik WS, Canakci H, Kilic IH, Celik F. Applicability of biocementation for organic soil and its effect on permeability. Geomech Eng. 2014;7:649–663.
117. Ivanov V, Chu J, Stabnikov V, Li B. Strengthening of soft marine clay using bioencapsulation. Mar Georesources Geotechnol. 2015;33:325–329.
118. Liu L, Liu H, Stuedlein AW, Evans TM, Xiao Y. Strength, stiffness, and microstructure characteristics of biocemented calcareous sand. Can Geotech J. 2019;56:1502–1513.
119. Smith AJ, Pritchard M, Bashir S. The reduction of the permeability of a lateritic soil through the application of microbially induced calcite precipitation. Nat Resour. 2017;8:337–352.
120. Fang C, Kumari D, Zhu X, Acal V. Role of fungal-mediated mineralization in biocementation of sand and its improved compressive strength. Int Biodeterior Biodegradation. 2018;133:216–220.
121. Omoregie AI, Ong DEL, Nissom PM. Assessing ureolytic bacteria with calcifying abilities isolated from limestone caves for biocalcification. Lett Appl Microbiol. 2019;68:173–181.
122. Helmi FM, Elmitwalli HR, Elnagdy SM, El-Hagrassy AF. Calcium carbonate precipitation induced by ureolytic bacteria Bacillus licheniformis
. Ecol Eng. 2016;90:367–371.
123. Dhami NK, Reddy MS, Mukherjee A.
Bacillus megaterium mediated mineralization of calcium carbonate as biogenic surface treatment of green building materials. World J Microbiol Biotechnol. 2013;29:2397–2406.
124. Kumar JPP, Babu RB, Nandhagopal G, Ragumaran S, Ramakritinan CM, Ravichandran V.
In vitro synthesis of bio-brick using locally isolated marine ureolytic bacteria, a comparison with natural calcareous rock. Ecol Eng. 2019;138:97–105.
Fig. 1A schematic illustration of soil biocementation using ureolysis-driven microbially induced carbonation precipitation method. 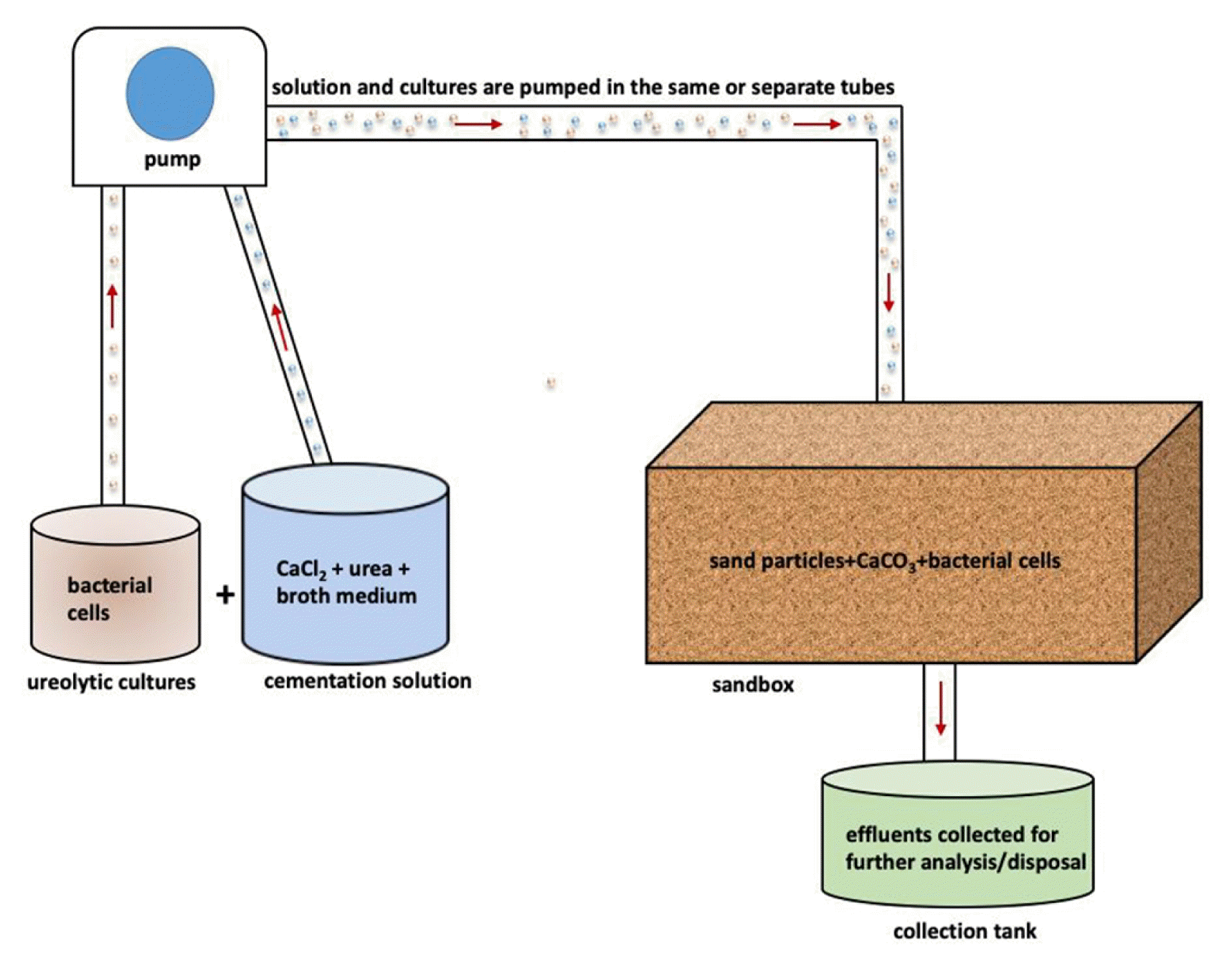
Fig. 2Process deliberation for soil biocement production using ureolytic bacterial cultures and cementation treatment solution. (a) Colonies of Sporosarcina pasteurii grown on an agar plate; (b) Broth containing ureolytic bacterial cultures grown overnight in conical flasks; (c) Calcium chloride flakes and granular urea powder; (d) Insoluble calcium carbonate precipitates formed after bacterial culture was inoculated into a solution containing cementation reagents [urea, CaCl2 and yeast extract]; (e) sandy soil prior to being immersed in polystyrene boxes; and (f) biocemented specimens placed outdoor to cure for some weeks after biocementation treatment with bacterial cultures and cementation solution is complete. 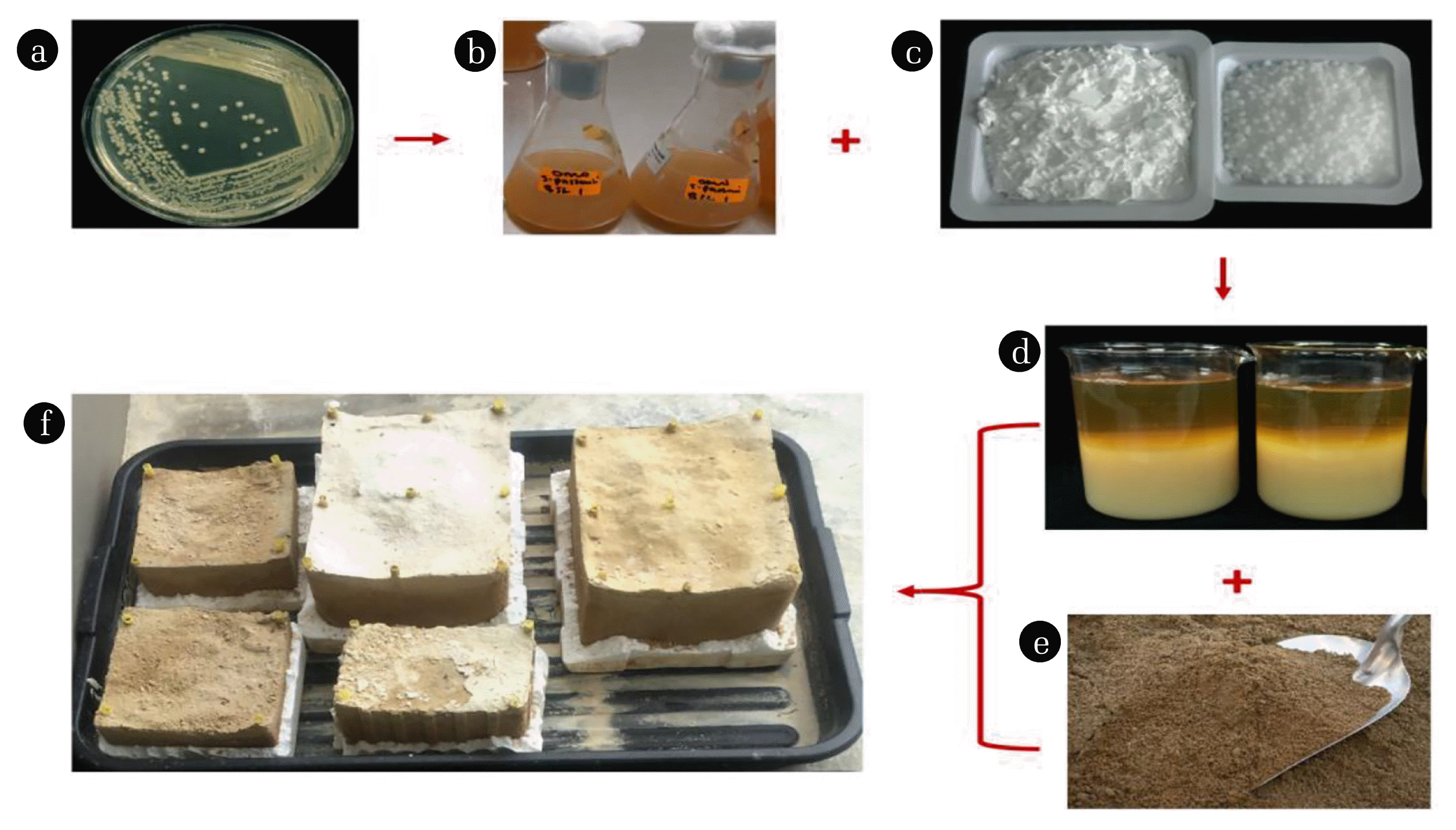
Fig. 3Bar charts showing an increment in the numbers of publications with keywords: “microbially induced carbonate precipitation”, “microbial urease” and “ureolytic” from 1999 to 2020. Data were sourced from the Scopus database on 16 June 2020. 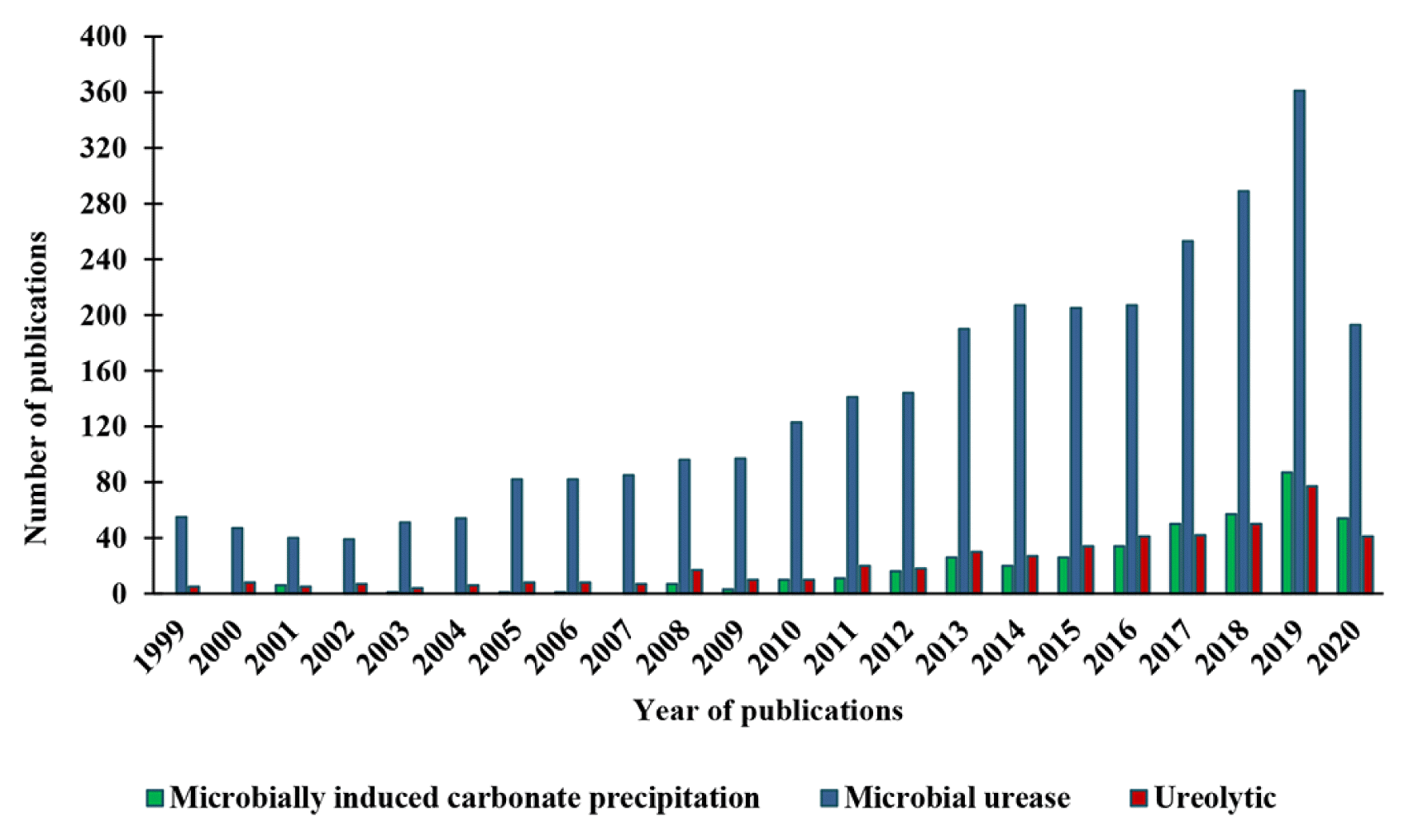
Fig. 4A representation of CaCO3 formation mediated by ureolytic bacteria (i.e. Sporosarcina pasteurii) through ureolysis-driven microbially induced carbonate precipitation pathway for soil improvement. The illustration was adapted from Liu et al. [114]. 
Fig. 5A conceptual diagram showing changes to soil grains during biocementation treatment. (a) Soil particles with pore space during cementation treatment; and (b) Soil particles with complete binding due to CaCO3 precipitation. The illustration was adapted from Naveed et al. [115]. 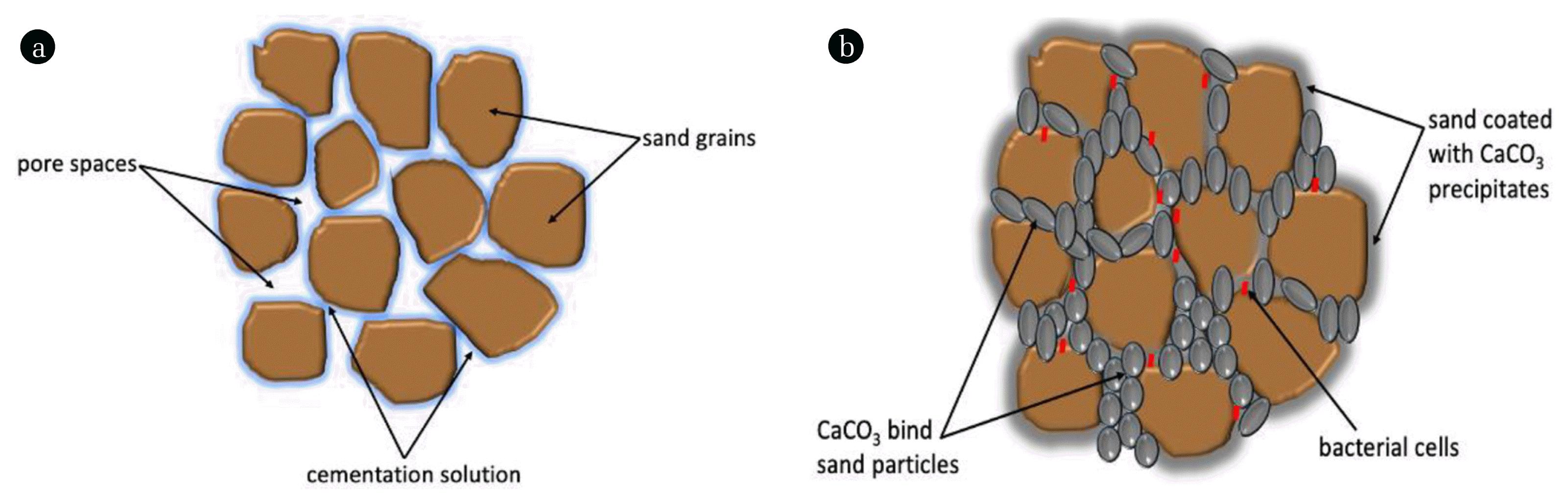
Table 1An Overview of Different Applications Using Ureolysis-driven Microbially Induced Carbonate Precipitation
Table 2Type of Soils Obtained from Various Locations Treated with Ureolysis-driven Microbially Induced Carbonate Precipitation
Table 3List of various ureolytic microorganisms isolated from various indigenous locations
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||