AbstractThe effects of environmental factors (temperature, humidity, and airflow) on gross calorific value (GCV) and proximate analysis of low grade coal were systematically investigated. The factorial experiments were conducted according to the statistical experimental design. The results were empirically modeled, statistically tested, and experimentally verified to quantify the change in GCV and proximate analysis of coal directed by environmental factors. The GCV of the coal were most affected by the humidity followed by the temperature. The GCV was increased from 5,365 to 5,986 (kcal/kg) through the decrease in humidity from 80 to 29 (% R.H.) and increase in temperature from 28 to 36 (°C). This increase in GCV could be attributed to the decrease in moisture content of the coal from 16.2 to 7.1 (%). Also, the optimized environmental conditions were identified where GCV ≈ 6,000 kcal/kg of the coal could be obtained. This study can be helpful in (i) managing the variability in GCV of a coal at different places, environment, and weather conditions and, thereby, settling the disputes between buyers and sellers over its variability and (ii) in maintaining the optimum conditions to enhance the GCV of the low grade coal in the power plants.
1. IntroductionCoal is the most consumed primary fossil fuel. Its consumption outranks by the factor of 3.4 and 3.8 times, respectively, when compared with the other two primary fossil fuels, i.e. oil and natural gas [1]. Moreover, its demand is predicted to increase over 50% in the coming decade [2]. According to the BP Statistical Review of World Energy 2019, the largest share i.e. ~38% of all the electrical power generation in the world is attributed to the coal [3]. In order to reduce the cost of electrical power generation, low grade coal characterized by the lower combustion value, is often used instead of high grade bituminous coal [4–6]. The South Korean domestic power plants, for instance, are reported to burn the coal having gross calorific value (GCV) of 5,400 kcal/kg in their power plants designed for 6,080 kcal/kg [6]. This use of low grade coal, in such power plants which are designed for the high grade coal, adversely affects the performance and economic life of auxiliary equipment (such as coal feeders, coal mills, and induced and forced draft fans, etc.) [6]. This problem can be addressed by managing the GCV of low grade goal.
The GCV, or higher heating value (HHV), is an indicator of chemically stored energy content of a coal [7, 8]. It is usually a decisive parameter in the assessment of the value of the coal and thereby provides a basis for its purchase agreement [9, 10]. GCV is the amount of energy released during the combustion of a coal. It is usually obtained by quantifying the total amount of heat evolved under the complete oxidation of a unit mass of a certain sample of coal in excessive amount of oxygen [11, 12]. The amount of heat evolved is measured, at specified conditions, using bomb calorimeter and expressed in BTU, MJ/kg, or kcal/kg [9, 13].
Thermal power plants often determine GCV and perform proximate analysis to assess the coals [8]. It is mainly because GCV provides quick and easy estimate of the quality of the coal [14]. Consequently, several equations have been developed over the years to predict the GCV of various classes of coals [8, 14–16]. Herein, we systematically studied the effects of environmental factors (temperature, humidity, and airflow) on GCV of low grade coal as characterized by the change in its moisture content, fixed carbon, volatile matter, and ash content (proximate analysis) using response surface methodology (RSM). RSM-based approach consists of the selection of appropriate statistical design of experiment, development of a mathematical model to represent experimental results, and quantitative representation of individual and combined effects of process factors on response [17, 18]. This approach substantially reduces the number of experiments, introduces the variability, and helps in quantitatively determining the linear, interaction, and quadratic effects of factors. It also provides a mathematical model for the response(s) of interest for prediction [17–20].
RSM had been successfully applied to improve the energy value of various organic wastes [21–24]. These studies were mostly focused on optimizing the experimental conditions to synthesize biofuel from agricultural wastes. However, to the best of our knowledge, no study is yet reported where GCV of commercial low grade coal is systematically investigated to characterize and optimize the calorific value of coal using RSM. The change in GCV of pulverized low grade coal, with respect to environmental factors, is the research question in our study. Though several factors affect the calorific value, including the type of coal, particle size, and environmental conditions [25–30]. The scope of this work is limited to explore the effect of environmental factors (temperature, humidity, and air flow) on low grade coal pulverized to obtain average particle size of 213 μm, according to standard method ASTM D7582-15 [31].
This study can be helpful in managing the GCV and proximate analysis of coal at different environmental conditions of temperature and humidity. Therefore, this study might be helpful in recommending environmental conditions to optimize the GCV of low grade coal for its use in the thermal power plants. Primarily, this work is aimed at resolving the conflict between buyers and sellers over the GCV of coal at various environmental conditions. Secondarily, provide scientific water spray strategy for reducing fine dust complaints. As most coal is more than a few centimeters in size and is transported by ship or train and stored in a coal yard, which can cause rare spontaneous coal combustion [32] and results in the increasing petitions from the nearby community on fine dust issues. So, water is sprayed regularly in the coal yard to protect the fine dust dispersion. In addition, to enhance the energy efficiency, pulverization process takes place just before being introduced into the boiler, and the pulverized coal is transferred to the air preheated to around 200°C and supplied to the boiler after a short residence time. Considering these conflict activities, our results can provide a practical scientific water spray management strategy to maintain GCV and protect fine dust at power plant.
2. Material and Methods2.1. MaterialsLow grade coal was acquired from Coal Indonesia Limited at Samarinda, East Kalimantan, Indonesia. The samples were pulverized and sieved to obtain particle size of ~213 μm mean diameter. The particle size distribution of coal is shown in Fig. S1.
2.2. Experimental SetupThe environmental conditions of temperature, humidity, and airflow were mimicked using a temperature, humidity, and airflow control chamber, PR-2J (Espec Europe GmbH, München, Germany). The samples were kept at specified conditions (of humidity, temperature, and air flow) in the chamber for a period of fifteen hours before analysis. The temperature was varied from 15 to 40 (°C), humidity from 20 to 80 (% relative humidity (R.H.)), and airflow below and above 0.5 m/s. These three experimental factors and their levels are shown in Table 1.
2.3. Sample AnalysesThe gross calorific values (GCV), proximate and ultimate analyses of the coal were performed. The gross calorific values were determined using Parr 6400 automatic isoperibol calorimeter manufactured by Parr Instrument Company (Moline, IL, USA) following the ASTM D5865/D5865M-19. Proximate analysis was carried out to determine the percentages of moisture, volatile matter (VM), fixed carbon (FC), and ash content using TGA701 Thermogravemetric Analyzer, manufactured by LECO Corporation (St. Joseph, MI, USA). Proximate analysis was performed under nitrogen atmosphere according to standard method, ASTM D7582-15. Ultimate analysis was performed using FLASH 2000 Elemental Analyzer, Thermo Fisher Scientific (Waltham, MA USA), and 5E-IRS3600 Automatic Infrared Sulfur Analyzer, CKIK (China). The elemental analyzer determined the content of carbon, hydrogen, and nitrogen whereas the sulfur analyzer quantified the amount of sulfur according to ASTM D 5373-16 and ASTM D4239-18, respectively. The oxygen content was approximated from the difference of all the elements and the ash from proximate analysis (i.e. O% = 100 – (C + H + N + S + Ash)%).
2.4. Experimental DesignCentral composite design (CCD) of experiments was selected for this study to comprehend, model, and optimize the effect of environmental factors on GCV and proximate analysis of low grade coal [33]. CCD is one of the most common types of statistical experimental design adopted in response surface methodology (RSM) to capture the effect of factors on response of interest [17, 18]. It provides the required information with fewer experiments. In case of three factors at five levels, the RSM required 24 experimental runs; eight at each of the central, factorial, and axial points in the experimental design space [19]. The central points provide the data regarding experimental error and reproducibility of the study. The suitable ranges of experimental factors, i.e. temperature, humidity, and airflow, were estimated from preliminary experiments. The preliminary experiments were done while changing one factor at a time. For instance, the effect of temperature was preliminary assessed by measuring mass loss over time through varying temperature while keeping all the other factors constant. The temperature and humidity were studied at five levels, whereas, the airflow was varied at two levels as shown in Table 1. The temperature and humidity were modeled as numeric (continuous) factors whereas the air flow was modeled as a categorical (either low or high) factor. The airflow was studied at two levels because the experimental chamber did not allow airflow velocity above 0.5 m/s. Moreover, it was not identified as a statistically significant factor during preliminary experimental investigations. However, it was included in the experimental design due to its practical importance because coal is usually stored in open yard and/or transported via ship in open.
The complete experimental design matrix exhibiting the combination of experimental factors and their responses is shown in Table 2. The response variables measured were GCV (kcal/kg), moisture (% mass fraction), fixed carbon (% mass fraction), volatile matter (% mass fraction), and ash (% mass fraction). The experiments were performed in random order to avoid systematic bias and minimize the effect of uncontrolled factors [17]. The models were developed by fitting the polynomial equations to the experimental data. The statistical significance and adequacy of the models were evaluated by analysis of variance (ANOVA) and determining their regression coefficients [19, 34].
3. Results and Discussion3.1. Characterization of Low Grade CoalThe GCV of the low grade coal, before exposing to specified environmental conditions shown in Table 2, was measured to be (5,327 ± 12) kcal/kg. The proximate analysis revealed 16.53% moisture, 37.37% volatile matter, 38.03% fixed carbon, and 7.68% ash as shown in Fig. S1. The ultimate analysis showed the elemental composition of the coal was as follows: 55% carbon, 5% hydrogen, 1% sulfur, 30% oxygen, and 1% nitrogen, also shown in Fig. S2. The effects of temperature, humidity, and airflow on GCV and proximate analysis of coal were recorded in Table 2. In general, the decrease in temperature and increase of humidly adversely affected the GCV of the coal. It was observed that the increase in GCV was neither directly proportional to the increase in the temperature, nor, inversely proportional to the decrease in the humidity of the air. Instead, it was a function of their synergistic combination. For instance, the highest GCV of the coal 5,986 kcal/kg (Exp. 12 in Table 2) was observed at 29% R.H. and 36°C, whereas, it was expected at 20% R.H. and 28°C (Exp. 1 in Table 2). The close examination of the experimental data emphasized the requirement of the mathematical modeling to accurately represent the effects of temperature and relative humidity on the GCV of the coal.
The decrease (increase) in the GCV of the coal, upon increasing (decreasing) the humidity in the air, can be attributed to the physical adsorption (desorption) of water molecules on the surface of the coal [25–27]. The increased amount of adsorbed water leads to the increase in the moisture content of the coal and ultimately results in the decrease of the GCV. Once the moisture content of the coal changes, its entire proximate analysis diversifies as shown in Table 2. Therefore, the quadratic equations were fitted to the experimental dataset for GCV (kcal/kg), moisture (%), fixed carbon (%), volatile matter (%), and ash (%), obtained after exposing the coal to various environmental conditions shown in Table 2.
3.2. Model Fitting and ANOVAThe empirical models, representing the effect of environmental factors on gross calorific value and proximate analysis of coal, are shown in Table 3. Eq. (1) mathematically represents the variability in the GCV of the coal as directed by the change in temperature (A) and humidity (B). Although, the airflow (C) was also attempted to model but its impact was statistically insignificant [17]. Therefore, it was not included in the final model (Eq. (1)). The equation (Eq. (1)), in its coded terms, can be helpful in estimating the relative impact of environmental factors on the GCV of the coal [18]. The impact of a certain environmental factor on the GCV can be estimated from the magnitude and symbol (+/−) of the coefficients in the equation [19]. The cursory glance at the Eq. (1) shows that the term A (temperature) has a synergistic whereas the term B (humidity) has an antagonistic effect on the GCV of the coal, represented by positive and negative symbols [35]. The synergistic effect means the GCV increases with the increase of temperature and antagonistic effect refers to the decrease in GCV with the increase of humidity. Also, the magnitude of the impact of humidity is 3.3 (205.59/62.05) times more than that of temperature. Eq. (2) is essentially the same as Eq. (1) except that it is corrected for actual terms. Therefore, Eq. (2) can be directly used to estimate the GCV of coal at various temperatures and humidity (% R.H.). Similarly, the empirical models representing the relationship between environmental factors and moisture, fixed carbon, volatile matter, and ash are represented by Eqs. (3), (5), (7), (9) (coded terms) and Eqs. (4), (6), (8), (10) (actual terms).
The statistical significance of the models was assessed by analysis of variance (ANOVA) [17, 18, 34, 36]. Table 4 shows the results of ANOVA, thereby, the statistically significant models and their terms. From Table 4, the F-value of 151.32 (along with p < 0.001) of the model representing the GCV of the coal implies that the model is significant. The linear terms of the model, A: temperature and B: humidity, are also highly significant (p < 0.001). Also, the interaction terms A2, B2, A2B, and AB2 are statistically significant at 94% confidence level. Only the term AB is statistically insignificant [18]. It is considered in the model to correct the hierarchy of the model since the A2B and AB2 are significant model terms. The R2 (0.985), adjusted R2 (0.979), and predicted R2 (0.960) values are consistently above 0.95 indicating the goodness of fit of the model [17–19]. The values of R2 and adjusted R2 are quite close (difference = 0.006) which indicates the significance of terms in the model. The adjusted R2 decreases if the added terms cease to add value to the model. The high value of predicted R2 and its close agreement with the adjusted R2 depicts the high predictability of the model. The high adequate precision value (34.85), signal to noise ratio, further adds to the confidence in the model [37]. The ANOVA results, therefore, statistically approve the model to represent the effect of environmental factors on the GCV of the coal. Similarly, the ANOVA analyses of the models developed to mathematically describe the effects of environmental factors on moisture, fixed carbon, volatile matter, and ash are statistically significant with reasonable regression co-efficient and adequate precision values as shown in Table 4. The experimental versus model predicted values are shown in Fig. 1. Fig. 1(a) shows the distribution of experimental vs model predicted GCV distributed around perfect prediction line (dotted line, RSM = experimental). A high correlation (R2 = 0.9849) is indicative of the robustness of the model. Similarly, the models developed for moisture (R2 = 0.9911), fixed carbon (R2 = 0.9633), volatile matter (R2 = 0.8387), and ash (R2 = 0.8499) correlate well with the experimental data as shown in Fig. 1. The ANOVA (Table 4) and correlation (Fig. 1) statistically established the models to be used to explore the effect of environmental factors on GCV and proximate analysis.
3.3. Influence of Temperature and Humidity on GCV
Fig. 2 shows the effects of environmental factors on GCV of the coal. The perturbation plot (Fig. 2(a)) shows the change in response as affected by the change in value of one factor at a time while keeping the other factors constant at reference point [17]. The reference point is coded 0 which corresponds to temperature (28°C), humidity (50% R.H.), and airflow (L, < 0.5 m/s) as shown in Table 1. It should be noted that the airflow failed to contribute significantly in the models (Eq. (1)–(10)), therefore, its level doesn’t matter. The plot shows the increase in GCV from 5,590 kcal/kg to 5,714 kcal/kg can be expected with the increase in temperature from 19°C (coded = −1) to 36°C (coded = +1) while keeping the humidity constant at 50% from Table 1. Also, the 5,634 kcal/kg of GCV can be expected at reference point (coded = 0) where temperature is 28°C and humidity is 50% R.H. The perturbation plot shows the prominent negative impact of humidity on the GCV of the coal. The GCV appears to decrease by 7% (5,862 – 5,449) kcal/kg with the increase in humidity from 29–71% R.H. increase in humidity while keeping the temperature constant at 28°C. This decrease in GCV of coal, upon increasing the humidity, can be attributed to the increase in moisture content of the coal at high humidity. The moisture content of the coal was determined by proximate analysis and discussed in subsequent section. This increase in GCV with the increase of temperature and the decrease in GCV with the increase of humidity are nearly linear as shown by the slopes of their respective lines in the perturbation plot. The contour plot (Fig. 2(b)) shows the GCV as affected by the temperature and humidity together, represented by the terms AB, A2B, AB2 in the model (Eq. (1)). The contours are not straight lines which shows the non-linear relationship between the GCV and combined effect of temperature and humidity. However, as shown in Fig. 2(a), (b), a pattern emerges indicating the gradual decrease in GCV with the increase of humidity at all studied temperatures.
3.4. Influence of Temperature and Humidity on Proximate Analysis
Fig. 3 shows the effects of environmental factors on proximate analysis of coal. The perturbation plots (Fig. 3(a), (c), (e), (g)) represent the impacts of an individual environmental factor (temperature or humidity) on the moisture, fixed carbon, volatile matter, and ash while keeping the other constant at center point (coded 0). The contour plots (Fig. 3(b), (d), (f), (h)) exhibit combined effects of environmental factors on proximate analysis of coal. Fig. 3(a) shows the minor (1.34%) decrease in moisture content (12.37% to 11.03%) of the coal with the increase of temperature from 19°C to 36°C. Whereas, the change in humidity from (29–71) % R.H. significantly (6.65%) increased the moisture content of the coal from 8.18% to 14.83%. This increase in moisture content adversely affected the GCV of the coal (Fig. 2). The combined effect of temperature and humidity on moisture content of the coal is shown by the contour plot in Fig. 3(b). Fig. 3(b) can be helpful in identifying the regions, shown by contour bands, where specific amount of moisture is most probable. For instance, the high moisture content (≥ 14%) can be expected at ≥ 60% R.H. and 18°C temperature, as well as, at 67% R.H. and 36°C temperature. Whereas, the low moisture content (≤ 8%) is probable only under 30% R.H. at 18°C or 36°C. Similarly, Fig. 3(c) shows the increase in fixed carbon content with the increase of temperature and decrease in fixed carbon with the increase of humidity. However, the effect of humidity is more pronounced indicated by the steeper slope of the line representing humidity. The adjacent contour plot (Fig. 3(d)) shows that ≥ 42% FC can be found when the humidity is ≤31% (at 18°C), ≤ 27% (at 27°C) or 36% (at 36°C). The volatile matter and ash content are affected by environmental factors in the similar fashion to that of fixed carbon. Fig. 3(e), (f) and Fig. 3(g), (h) show the effects of environmental factors on volatile matter and ash content, respectively.
3.5. OptimizationExperiments were conducted for the optimization of environmental factors to maximize the GCV of the coal. It will be helpful in designing the coal storage yard to derive maximum heat energy from the coal. The significance of optimization is to determine the environmental conditions to for pre-drying the low grade coal for use in place of high grade coal in power plants. The optimization was performed around a single parameter; i.e. GCV maximization while scanning through various combinations of environmental factors. The desirability function was employed to perform numerical optimization which was developed by Myers et al. [17]. The details about the desirability function can be found in our earlier works [34, 37]. Fig. 4(a) shows the ramp plots indicating the optimum environmental factors (red dots) resulting in the maximum GCV (blue dot) of the coal and corresponding proximate analysis (grey dots). The location of optimum parameters is shown by a flag in the response surface plot in Fig. 4(b). It is shown that a GCV of 6,013 kcal/kg could be achieved by keeping the sample at 35°C and 20% R.H. The experiment was performed at optimized conditions which yielded the GCV of 5,970 kcal/kg, i.e. 0.7% less than the predicted value. It exhibits the very good predictability of the model. Table S1 (in supporting information) shows the comparison of RSM model predicted (Eq. (1)–(10)) values compared with the experimental values of GCV and proximate analysis. The % error indicates the reasonable prediction of GCV and proximate analysis of the coal by the RSM models. The models, therefore, can be reliably used to predict the GCV, moisture content, fixed carbon, volatile matter, and ash content of the low grade coal at various environmental conditions.
4. ConclusionsResponse surface methodology (RSM) is a useful tool to model, predict, and optimize the change in the GCV and proximate analysis of low grade coal with the change of the environmental conditions. It was observed that the GCV and proximate analysis of the low grade coal significantly varies with the environmental conditions such as temperature and humidity. Approximately 621 kcal/kg difference in GCV was observed as the temperature and humidity were varied between 28 to 36 (°C) and 29 to 80 (% R.H.), respectively. The considerable decrease in GCV of the coal (with the increase of humidity) can be attributed to the increase in moisture content of the coal. The moisture content of the coal increased from 7.14 to 16.18 (%) as the humidity increased from 29 to 80 (% R.H). resulting in the decrease of GCV from 5,986 to 5,365 (kcal/kg). The optimized environmental conditions were predicted, and later experimentally verified, to maximize the GCV of low grade coal. It was observed that the GCV of the low grade coal can be increased to ~6,000 kcal/kg (from ~5,300) by storing it at optimized environmental conditions, i.e. 35°C and 20% R.H. A fifteen hours’ exposure of the pulverized coal, at optimized environmental conditions, may increase ≤ 13% GCV of a coal by reducing its moisture content by ≤ 56%. This study can be helpful in managing the GCV of low grade coals and tailoring them for intended use.
AcknowledgmentThis work was supported by the Korea Institute for Advanced of Technology (KIAT) grant funded by the Korea Government (MOTIE) (P0008421, The Competency Development Program for Industry Specialist). The authors would like to express sincere gratitude to Intertek Testing Services Korea Ltd. for supporting all experimental and analytical facilities.
NotesAuthor Contributions S.P. (Ph.D student) designed, carried out the experiments, analyzed the experimental results and lead in draft writing the manuscript. Q.Z. (Post-doc) supported statistical data analysis and paper writing. H.P. (Professor) was in charge of overall direction, planning and final approval. All authors provided critical feedback and helped shape the research, analysis and manuscript. References1. Adiansyah JS, Haque N, Rosano M, et al. Application of a life cycle assessment to compare environmental performance in coal mine tailings management. J Environ Manage. 2017;199:181–191.
3. Dudley B. BP statistical review of world energy. BP Statistical Review; London, UK: 2019.
4. Luo Z, Agraniotis M. Low-rank Coals for Power Generation, Fuel and Chemical Production.
5. Zhang D, Jackson PJ, Vuthaluru HB. Low-Rank Coal and Advanced Technologies for Power Generation. Impact Miner Impurities Solid Fuel Combust. 2005;45–64.
6. Kim H, Kim B, Byun SH. Optimization of calorie compensation of fuel coal for power plant control. In : 37th Annual Conference of the IEEE Industrial Electronics Society; 7–10 Nov 2011; Melbourne. p. 902–905.
7. Mesroghli S, Jorjani E, Chehreh Chelgani S. Estimation of gross calorific value based on coal analysis using regression and artificial neural networks. Int J Coal Geol. 2009;79:49–54.
8. Majumder AK, Jain R, Banerjee P, et al. Development of a new proximate analysis based correlation to predict calorific value of coal. Fuel. 2008;87:3077–3081.
9. Chelgani SC, Hart B, Grady WC, et al. Study relationship between inorganic and organic coal analysis with gross calorific value by multiple regression and ANFIS. Int J Coal Prep Util. 2011;31:9–19.
10. Matin SS, Chelgani SC. Estimation of coal gross calorific value based on various analyses by random forest method. Fuel. 2016;177:274–278.
11. Llorente MJF, García JEC. Suitability of thermo-chemical corrections for determining gross calorific value in biomass. Thermochim Acta. 2008;468:101–107.
12. Patel SU, Jeevan Kumar B, Badhe YP, et al. Estimation of gross calorific value of coals using artificial neural networks. Fuel. 2007;86:334–344.
13. Montgomery WJ. Standard Laboratory Test Methods for Coal and Coke. Anal Methods Coal Coal Prod. 1978;191–246.
14. Parikh J, Channiwala SA, Ghosal GK. A correlation for calculating HHV from proximate analysis of solid fuels. Fuel. 2005;84:487–494.
15. Mott R, Spooner C. The Calorific Value of Carbon in Coal: The Dulong Relationship. Fuel. 1940;19:226–231.
16. Given PH, Weldon D, Zoeller JH. Calculation of calorific values of coals from ultimate analyses: theoretical basis and geo-chemical implications. Fuel. 1986;65:849–854.
17. Myers RH, Montgomery DC, Anderson-Cook CM. Response Surface Methodology : Process and Product Optimization Using Designed Experiments. John Wiley & Sons; 2016.
18. Anderson MJ, Whitcomb PJ. RSM simplified: optimizing processes using response surface methods for design of experiments. Productivity press; 2016.
19. Anderson MJ, Whitcomb PJ. DOE Simplified: Practical Tools for Effective Experimentation. Productivity press; 2015.
20. Anupam K, Sikder J, Pal S, et al. Optimizing the cross-flow nanofiltration process for chromium (VI) removal from simulated wastewater through response surface methodology. Environ Prog Sustain Energy. 2015;34:1332–1340.
21. Anupam K, Sharma AK, Lal PS, et al. Preparation, characterization and optimization for upgrading Leucaena leucocephala bark to biochar fuel with high energy yielding. Energy. 2016;106:743–756.
22. Chiou B, Sen Valenzuela-Medina D, Bilbao-Sainz C, et al. Torrefaction of almond shells: Effects of torrefaction conditions on properties of solid and condensate products. Ind Crops Prod. 2016;86:40–48.
23. Lee JW, Kim YH, Lee SM, et al. Optimizing the torrefaction of mixed softwood by response surface methodology for biomass upgrading to high energy density. Bioresour Technol. 2012;116:471–476.
24. Pinto F, Hidalgo-Herrador JM, Paradela F, et al. Coal and waste direct liquefaction, using glycerol, polyethylene waste and waste tyres pyrolysis oil. Optimisation of liquids yield by response surface methodology. J Clean Prod. 2020;255:120192.
25. Pang S, Bhattacharya S, Yan J, et al. Advances in Coal Drying. Dry Biomass, Biosolids, Coal. 2019;135–164.
27. Karthikeyan M, Zhonghua W, Mujumdar AS. Low-rank coal drying technologies - Current status and new developments. Dry Technol. 2009;27:403–415.
28. Jangam SV, Karthikeyan M, Mujumdar AS. A critical assessment of industrial coal drying technologies: Role of energy, emissions, risk and sustainability. Dry Technol. 2011;29:395–407.
29. Sun Y, Xu C, Xin T, et al. A comprehensive analysis of a thermal energy storage concept based on low-rank coal pre-drying for reducing the minimum load of coal-fired power plants. Appl Therm Eng. 2019;156:77–90.
30. Edward KL, Nenad S, Harun B, et al. Use of coal drying to reduce water consumed in pulverized coal power plants. 2006;
31. ASTM D 7582-15. Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM Int. 2015;
32. Merwe D, Campbell QP. An investigation into the moisture absorption properties of thermally dried South African fine coal. J South Afr Inst Min Metall. 2002;102:417–420.
33. Omulo G, Banadda N, Kabenge I, et al. Optimizing slow pyrolysis of banana peels wastes using response surface methodology. Environ Eng Res. 2019;24:354–361.
34. Zaib Q, Ahmad F. Optimization of Carbon Nanotube Dispersions in Water Using Response Surface Methodology. ACS Omega. 2019;4:849–859.
35. Anupam K, Deepika , Swaroop V, et al. Antagonistic, synergistic and interaction effects of process parameters during oxygen delignification of Melia dubia kraft pulp. J Clean Prod. 2018;199:420–430.
Fig. 1Experimental vs RSM model predicted values of (a) GCV, (b) Moisture, (c) Fixed carbon, (d) Volatile matter, and (e) Ash. The dotted lines (RSM = Experimental) pass from the origin and represent the perfect prediction. 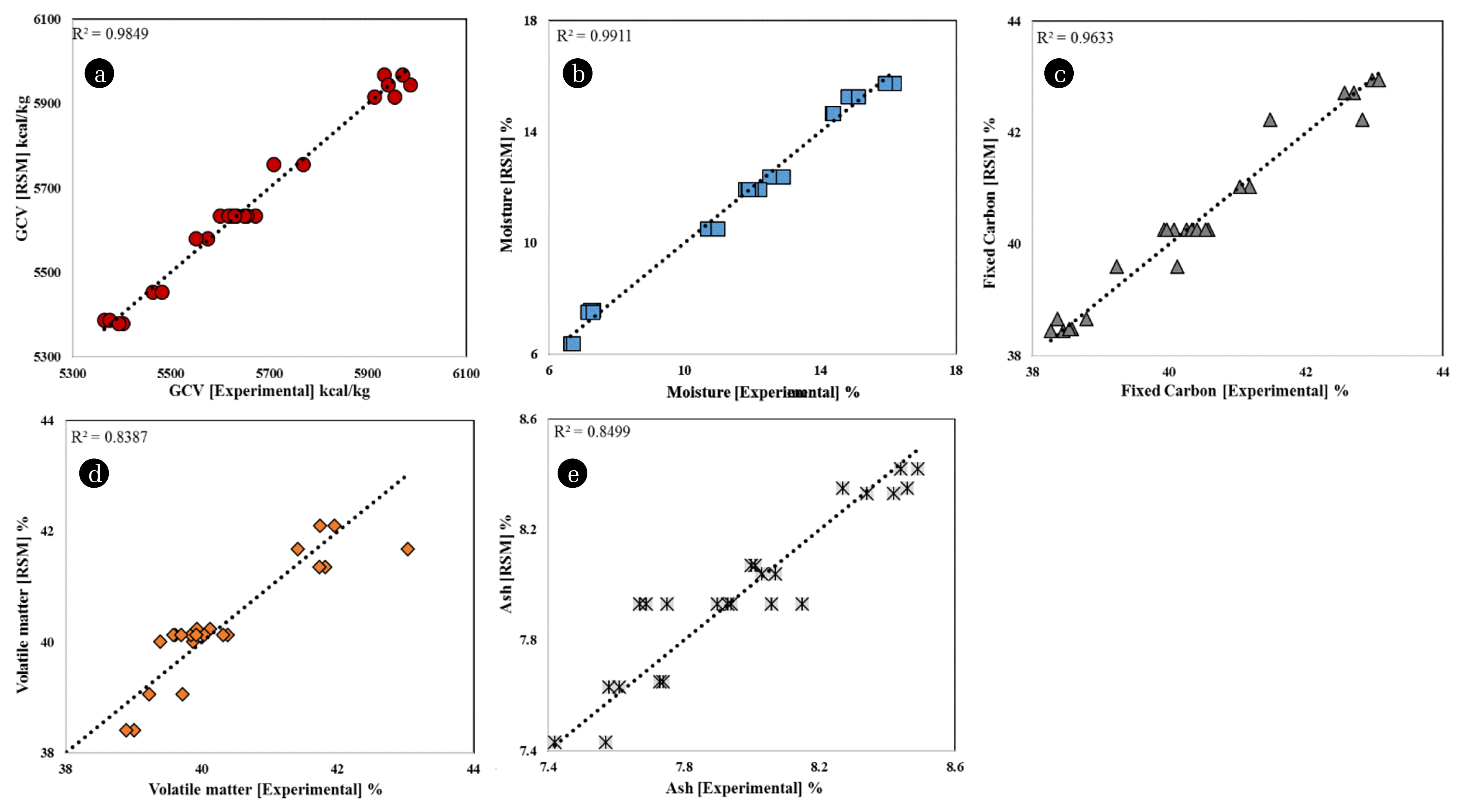
Fig. 2(a) Perturbation plot and (b) Contour plot representing the impacts of temperature and humidity on the GCV of low grade coal. 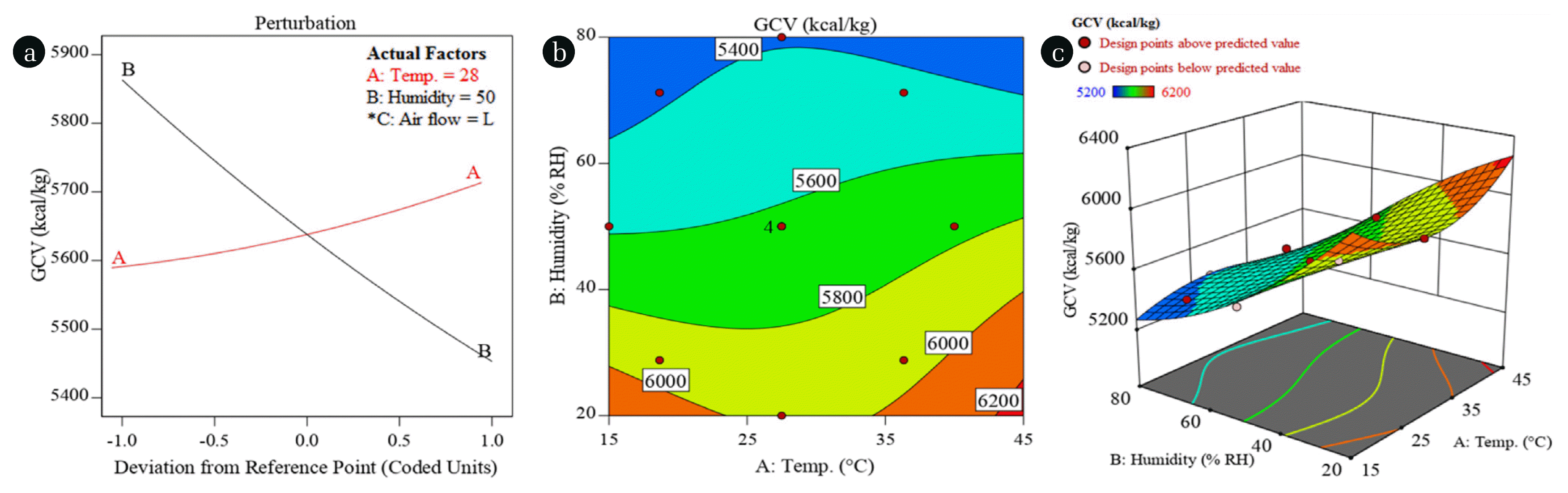
Fig. 3Perturbation plots and contours representing the impacts of temperature and humidity on proximate analysis of low grade coal. (a), (b) Moisture, (c), (d) Fixed carbon, (e), (f) Volatile matter, and (g), (h) Ash. 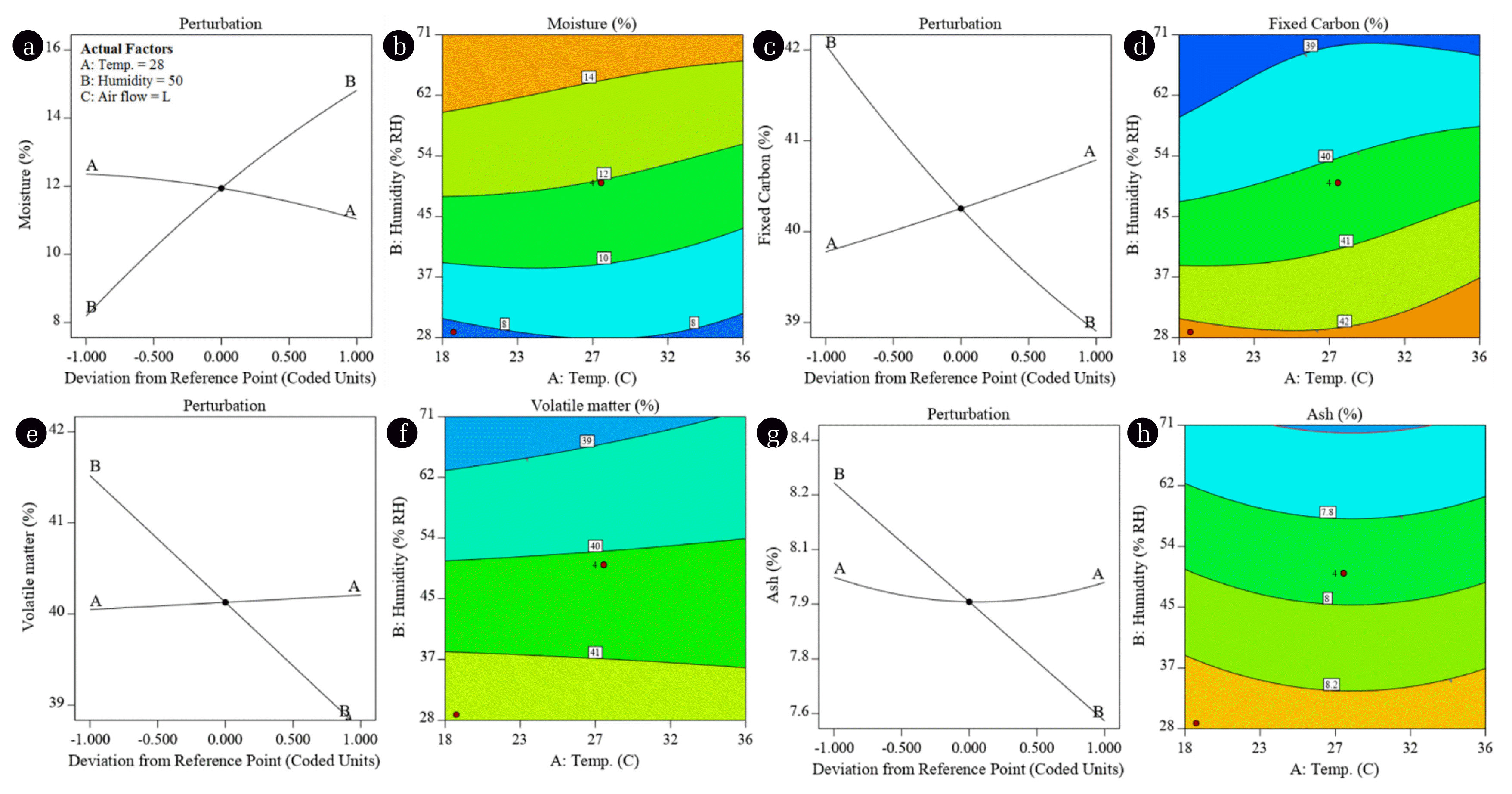
Fig. 4(a) Ramp plots indicating the position of factors (red) and response (blue) at optimized conditions. (b) Response surface representing the location of optimized point (flag) where the highest GCV can be achieved. 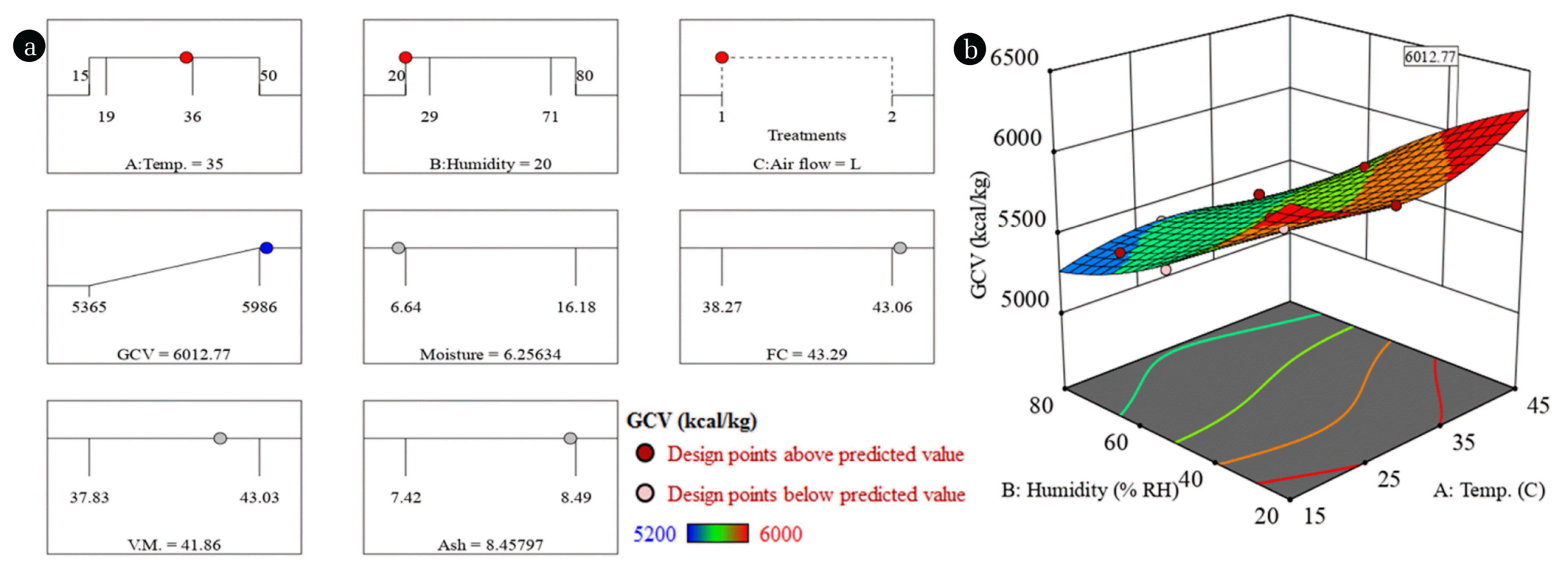
Table 1Experimental Design Factors and Levels
Table 2The Experimental Results Obtained from Various Experimental Setting of Environmental Factors Table 3Models Representing Gross Calorific Value and Proximate Analysis of Low Grade Coal Table 4ANOVA Results and Adequacy of the Models for the GCV and Proximate Analysis of Low Grade Coal |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||