AbstractThe natural Fly ash modified with calcium oxide has been employed to eliminate the crystal violet dyestuff from the simulated solution. Herein, the effect of different physicochemical factors like primary dye concentration, sorption contact time, the quantity of the adsorbent, temperature, along with initial simulated solution pH, evaluated for illustrating the mechanism of adsorption. Furthermore, the equilibrium study was conducted, and equilibrium models like Langmuir, Freundlich, and Dubinin-Raduskevich (D-R) were fitted to obtain analytical results to endow with more insight into the process. The results acknowledged that the Langmuir model is well apt and suggests that the adsorption mechanism happens in a monolayer on the fly ash surface. Pseudo-first order, Pseudo-second order, and the intraparticle diffusion model evaluated, and the interpretation suggests the sorption method is obeying the Pseudo-second order and intraparticle diffusion model. The ascertained negative values of Gibbs free energy affirmed the unconstrained process for all symbiotic associations, and the obtained data 78.70 kJ mol−1 enthalpy values manifested that exothermic mechanism was governing the reaction. The above assessment confirms the application of Calcium oxide pre-treated fly ash as a cheap adsorbent to eliminate the crystal violet dyestuff from the simulated solution.
1. IntroductionThe rapid progressive urban and industrial expansion in modern society developed a severe threat to human health, degrading the quality of water in numerous ways. The continuous increment of the pollution gave rise to an alarming stage in the present society. Although various pollutants are present, amongst them, dyes are significant key players that create unsafe situations of water pollution in the environment [1]. Dye possesses several applications in a wide span of industrial applications. Since its inception of use ubiquitously in different industries such as textile, coloring material agents, and pharmaceuticals, it’s omnipresent nature. Dyes consider as the most adverse ecotoxicological material [2]. The minute presence of the dye molecules is highly detrimental [3]. It is evident from the literature that around 10,000 different types of dyes exist commercially. The primary usage of dyes is ubiquitously present in textile, paper, plastics, leather, pharmaceutical, food industry, etc. [4–6]. The rate of production of the dyes 7 × 105 to 1 × 106 tons per year [7, 8]. It is manifest that from the literature, around 10–15% of dyes entered the natural ambiance without prior treatment and posed some grave consequences to the ambiance [8]. The textile industry plays a primary role in environmental pollution due to the massive discharge of wastewater containing a high concentration of dye as effluent. The presences of these chemicals are solely responsible for the destruction of the aquatic ecosystem. Not all the dye molecules incorporated for the dying process is utilized. The available dye molecules are often exposed to the environment causing hazardous impact. The fabric industries consume about 90% of the total dyes manufactured. For wet processing in the fabric industry, a considerable amount of water is required, and a significant amount of un-utilized dyes, acids, alkalis etc. are produced. The cost of restoration is much higher and demands suitable, sustainable technology for remediation. Total dissolved solids in the effluent wastewater should not exceed 110% i.e., above 13–14 mg L−1. In Bangladesh, the total solids concentration in discharge is limited to 2100 mg L−1 [9] above this permissible limit, the presence of dyes can eventually harm the aquatic life [10]. Due to the unwanted entry into a natural ambiance, the balance of the ecosystem gets disturbed and generates a high risk of causing several dreadful diseases and initiation of health hazard [11, 12]. The disorders which have been diagnosing due to this effect of these dyes are such as skin allergy, dermatitis, and cancer [13, 14]. Sometimes, due to the higher concentration of dye sunlight cannot penetrate the subsurface of the water in the natural streams, lakes, and rivers create a negative impact on the aquatic life/ phytoplankton and zooplanktons [15].
Crystal Violet (CV) a triphenylmethane dyestuff, employed in apparel and fabrics for coloring the material. In printing and paint industry used this dye [16]. CV ubiquitously used in the medical community. The CV has numerous effects that create several health disorders. [16]. The eradication or elimination of CV dyestuff from the water bodies is exceptionally vital for the betterment of society. Herein, we have attempted to eradicate CV dye from the simulated solution with the aid of modified fly ash.
It is challenging to dispose of the dye-containing effluent without proper treatment, mainly due to the inherent property of the synthetic dyes. Owing to the multifaceted molecular arrangement of the dyestuff, they are unable to degrade naturally [11, 16, 17]. There are some of the efficient and accessible technologies used presently for the removal of dyes, which include filtration technologies like ultrafiltration [18], nanofiltration [19], reverse osmosis [20]. But filtration technology exhibits certain major drawbacks like clogging of pores, membrane denaturation etc. Other than these several traditional technologies have been adopted for the eradication of the dyestuff such as photo-catalysis, Solar Photo-Fenton, biochemical degradation, etc. [6]. Lately, a technique of molecular imprinting is developed where template molecules are removed at different polymerization stages [21] the process is precise. Although, all the methods mentioned above are not fully effective and possess cons such as partial elimination, engrossment of expensive chemicals, production of hazardous substrate creates secondary issues like careful removal, massive investment, the involvement of substantial human resources, etc. Compare to traditional treatment methods of dyestuff contaminated effluents, sorption has to appear as a simple and effective process [22]. Adsorption is a technique in which a substrate coheres upon the adsorbent from its ambiance [23, 17]. This alternative method of separation in environmental engineering has gained immense importance and is now taken into consideration worldwide for its versatility [24]. Many natural materials or by-products of industries can be either used directly or after minor pre-treatment to eliminate the CV dye from the simulated solution [25, 7, 17]. Herein, this piece of the work deals with the utilization of natural fly ash and calcium oxide modified fly ash for the elimination of cationic dye like Crystal violet used in this study. The utilization of the fly ash caters to two aspects, like removal of the dye-containing water along with the possible usage of solid waste generated in the power industry where coal used as raw material.
Fly ash is a secondary product of coal utilizing energy plants. But effective disposal of fly ash is still a significant challenge, and it is an absolute menace to the environment as a pollutant [26]. Fly ash is a pozzolanic substrate consists of SiO2, CaO, and Al2O3. Fly ash has a universal application as a core substrate for sorption studies. The fly ash showed striking competencies for sorption studies regarding economic feasibility and environmental perspectives [25, 27]. Highly porous adsorbents with a significant surface area showed enhanced adsorbing capacity. Hence, fly ash has modified with calcium oxide (CaO) for the increment of its sorption capacity [28]. It is evident from the literature that hazardous gases such as SO2, CO2 could be entrapped with the aid of alkalinity increment of the fly ash [29]. Batch studies have performed, and the effect of the factors has evaluated. The role of primary dye concentration, sorption contact time, the quantity of the adsorbent, temperature, initial simulated solution pH, and agitation speed on CV sorption analyzed in this investigation. Further, an equilibrium study has been carried out employing Langmuir, Freundlich, and Dubinin- Raduskevich (D-R) equilibrium models [30] Furthermore, the kinetic parameters have been assessed by Pseudo-first order, Pseudo-second order and intraparticle diffusion model [31]. The activation energy (Ea) for the sorption mechanism determined by the Arrhenius equation. Additionally, thermodynamic factors such as Gibbs free energy (ΔG°), enthalpy (ΔH°), entropy (ΔS°), and Isosteric heat was determined to apply the Clausius-Clapeyron equation [32]. The entire above factors are essential for comprehending the nature of adsorption.
2. Material and Methods2.1. Collection and Modification of Pristine AdsorbentThe natural fly ash collected from the local coal-based energy plants. Initially, after the collection of the fly ash rinsed extensively with the deionized water to eradicate the unwanted coarse particles. After rinsing, the wet fly ash sample has been dried for 24 h in a Hot Air oven at a temperature of 343 K. Further, the dried fly ash sample was subjected to exposure at 5% CaO. The sample solution was kept in an autoclave for 15 min at 10 psi. Furthermore, the suspension was purified by filtration rinsing with deionized water until the pH of the treated fly ash sample reached neutral. Additionally, after neutralization by rinsing, the modified fly ash sample was again kept for drying at 343 K for 24 h. Finally, after drying the treated fly ash was ready to use for the sorption experiments.
2.2. Simulated Dye SolutionsThe procured CV employed in the experiments was ultra-pure and used with no further purification. The configuration of the CV exhibited in Fig. S1. The properties of CV employed in the study provided in Table S1. The stock solution of crystal violet (500 mg L−1) made by weighing the exact measure of the dye in the deionized water. The working solution of different concentrations was made from stock with further dilution from stock solution applying the precise volume of deionized water.
2.3. Batch ExperimentsThe batch study has performed in Erlenmeyer flasks (250 mL) with the cap. A primary concentration of 50 mg L−1 with an adequate volume of 100 mL was used. Further, a predetermined quantity of the sorbent mixed into the dye simulated solution. The flask was stirred at a specific speed of 140 r/min for 3 h in a shaker incubator at a constant temperature 303 ± 1 K, the role of the different experimental factors, was determined such as primary pH in the range of (2–10), primary dye concentration (10–100 mg L−1), sorption contact time (10–180 min), the quantity of the adsorbent (0.5–5 g L−1), and temperature (303–333 K). The dye solution was collected from the Erlenmeyer flask at a particular period for further analysis of the remaining dye concentration in the solution. Furthermore, the collected sample has been subjected to centrifugation at a specific speed of 4000 r/min intended for a set time of 10 min. The residual dye concentration analysed by using the UV-VIS Spectrophotometer at 580 nm (Model SP7576–Spectrum, Shanghai, PR China) after a specific time interval. The amount of the dye adsorbed for every unit of the adsorbent (“mg” dye / “g” sorbent) was determined based on the mass balance of the dye retained within the primary solution applying Eq. (1):
To determine the removal percentage (%) of the dye from the simulated solution was evaluated by applying the Eq. (2):
Experiments have been performed in triplicate to eradicate any errors, and further, the average values have applied for the calculation of experimental data. In our study, we have performed control experiments by incorporating the adsorbent, affirmed the adsorption of the dyestuff on the internal wall of the flasks above was minuscule.
3. Theory3.1. Equilibrium AnalysisHere in this study, equilibrium association among the sorbate concentration in liquid ambiance and sorbent surface in a simulated ambiance has been deciphered with Langmuir, Freundlich, and Dubinin-Raduskevich (D-R) equilibrium models. [30, 32]. The mathematical expressions of the Langmuir, Freundlich, and Dubinin-Raduskevich (D-R) equilibrium models are tabulated in Table S2. The Freundlich equilibrium model is more suitable for non-absolute sorption on the composite surface.
3.2. Adsorption KineticsHerein, these kinetic study models have been employed in the study, for instance, Pseudo-first order, followed by Pseudo-Second order and Intra-particle diffusion model. The model, as mentioned above, has been determined by the mathematical expression represented in Table S2.
3.3. Activation Energy and Thermodynamic ParameterThe activation energy (Ea) of the present study was determined by using the Arrhenius equation tabulated in Table S2.
4. Results and Discussion4.1. Adsorbent Characterization: Scanning Electron Microscopy AnalysisThe natural and modified fly ash adsorbent was characterized by Scanning Electron Microscopy (SEM) analysis for understanding the surface quality of the sorbent. The images of the SEM have captured with the help of SEM (Model S-3000N, Hitachi, Japan). Preceding to the scanning of the sorbent was layered with a fine layer of gold to provide the sample enriched with electrons or enhance the conductivity of the sample. The surface quality of the fly ash has investigated by SEM. The surface quality of the raw fly ash consists of several pores. After treatment with CaO, the pore has been broadened, and after adsorption of the dye molecules, the vacant pore has been filled with adsorbed dye molecules, which illustrated in Fig. S2.
4.2. Adsorbent Characterization: XRD CharacterizationThe crystalline structure of the fly ash samples was evaluated using the Rigaku X-ray diffractometer (XRD) with Cu-Kα radiation, over 2θ range 20–80°. The primary constituent in the fly ash samples is silicon-aluminous material, as confirmed by XRD spectra, Fig. 1. The major crystalline mineralogical constituents of the fly ash sample used in this study are: quartz (SiO2), sodium aluminium silicate (Na(AlSi2O6)), tricalcium magnesium orthosilicate (Ca3Al2(SiO4)2), magnesium silicate (Mg2SiO4), mullite (Al4Si2O10), anhydrite and hematite (Fe2O3) [33]. The XRD data show that new alumino-silicates with higher peak areas are formed due to calcium oxide treatment of fly ash [34]. An increase in the crystallinity due to the alkaline chemical treatment confirms restructuring within the fly ash substrate that enhances adsorption efficiency. Along with an extensive and irregular background in the X-ray diffractogram of the fly ash data, we also observe an abundance of glassy and amorphous constituents.
4.3. Effect of Influencing Factors4.3.1. Effect of pH of primary solutionThe pH plays a substantial role in the sorption of the dye molecules. The surface charge of the molecules has been affected by the changes in the pH of the sorbate. This phenomenon might be happening due to the degree of ionization of the dyestuff [32]. For conformational stability and color, potency pH acts as a significant factor. The pH controls the competition among the hydrogen ions and the dye molecules on the active sites of the sorbent surface. In the present study, the pH range varied between 2 – 10 and elucidated in Fig. 2(a). The initial pH of the system was varied, keeping other operating experimental conditions constant at primary dye concentration: 50 mg L−1, amount of adsorbent 2 g L−1, temperature: 303 K, agitation speed: 140 r/min, sorption contact time 3 h. CaO modified fly ash seemed to be competent to remove the CV from the simulated solution. It was observed from the experimental data that higher removal percentage achieved with increasing pH up to 8. Further, an increment in the pH up to 10 was not favorable in removing the dye, as mentioned earlier. Later on, pH 8 was the optimized value obtained from the experimental results.
The CV is a basic dye, present as positively charged ions in the simulated solution. At lower pH, existing of the positively charged functional group on the sorbent surface repels the positively charged dye molecules, resulting in low sorption of the dye molecules in highly acidic ambiance. Contrastingly, in the basic ambiance, the phenomenon above is vice-versa, resulting in adherence of the dye molecules to the sorbent surface. It is evident from the literature that increment in pH value ascribed to lowering H+ ions on the sorbent surface, resulting in a high percentage of dye removal at higher values of the pH. In contrast, at low pH values, H+ ions actively participate on the sorbent surface, resulting in lowering the dye removal efficiency. A similar trend observed for adsorption of CV onto sodium hydroxide modified rice husk [31].
4.3.2. Effect of amount of adsorbent
Fig. 2(b) deciphered the sorption of CV Vs. Pristine and a modified fly ash quantity of adsorbent ranges from 0.5–5 g L−1. The initial adsorbent loading of the system was varied, keeping other operating experimental conditions constant at primary dye concentration: 50 mg L−1, pH of primary solution: 8.0, temperature: 303 K, agitation speed: 140 r/min, sorption contact time 3 h. Fig. 2(b) reveals that while raising the quantity of the adsorbent percentage of elimination too increased simultaneously. This kind of phenomenon happened might be owing to the numeral of accessible pores existing on the facade of the adsorbent. From the literature, it has suggested that an increase in the amount of adsorbent might create numerous vacant active sites [31, 30]. Although, we came across the fact that the simultaneous rise in the quantity of the adsorbent will enhance the removal percentage of the CV from the simulated solution.
In contrast, increasing the percentage removal while increasing the amount of adsorbent leads to a decrease in the adsorption capacity, an inverse relationship has detected. This kind of phenomenon might be happening due to the unavailability of the pores on the facade of the adsorbent or owing to the aggregation of the filling of the active sites [31]. A significant reduction in qe value was observed to rise in the concentration of the sorbent mass. The phenomenon might be due to a decrease in the unit mass adsorbed onto the surface of the adsorbent. Later, the highest removal percentage of the dyestuff recorded was 97.86 % at 2.0 g L−1. However, there was no promising result achieved by exceeding the amount of adsorbent. This phenomenon might be due to the equilibrium achieved among dyestuff molecules adhered to the sorbent surface and the simulated solution [32]. Finally, after the evaluation of the sorption process, 2.0 g L−1 of the amount of sorbent was considered as an optimized mass and further employed in the analysis of other influencing factors.
4.4.3. Effect of primary dye concentration and sorption contact timeThe primary dye concentration plays an essential function in the sorption investigation. The role of varying the primary concentration of CV onto the modified fly ash and natural fly ash showed in Fig. 2(c). The effect of primary dye concentration was studied, keeping other operating experimental conditions constant at the amount of adsorbent: 2 g L−1, pH of primary solution: 8.0, temperature: 303 K, agitation speeds: 140 r/min, sorption contact time 3 h. It is evident from the analytical data that a higher concentration of the adsorbate solution leads to a decreasing in the percentage removal of the CV dye from the simulated solution. The justification for this kind of behaviour might be due to the lack of active sites for accommodating another dye molecule in its active site. Nevertheless, the sorption capacity at stability is positive, with an augmentation in the dye concentration. These might be an increase in a concentration gradient, which serves as enhancing the driving force to repress all mass transfer hindrances of the CV among the liquid and solid-state, notably enhancing isothermal sorption until the adsorbent saturation has gained.
The contact time between sorbent and sorbate also played an essential role in the sorption of CV. It has investigated from the obtained data that sorption of CV onto the CaO modified fly ash surface played a decisive role with increasing contact time until 120 min. The sorption reached equilibrium after 120 min. Therefore, no significant increase observed after 120 min. Rapid adsorption that happened at the beginning step might be justified due to the presence of vacant pores upon sorbent surfaces. The reason behind the fast adsorption that occurred in the early stage might be due to the diffusion mechanism. Later on, the adsorption probably an adhered governed process might be owing to the few vacant sites.
4.3.4. Effect of temperatureTemperature plays a crucial role in the adsorption process. The rise in temperature varies the isothermal capacity of the sorbent for a specific dye. Hence, the role of temperature in the present study has investigated. Fig. 2(d) showed the role of temperature on the sorption of CV employing the CaO modified fly ash. The effect of temperature was studied, keeping other operating experimental conditions constant at primary dye concentration: 50 mg L−1, amount of adsorbent: 2 g L−1, pH of primary solution 8.0, agitation speed: 140 r/min, sorption contact time 3 h. It reveals from the figure that the removal percentage declined with rising in temperature up to a certain level. It observes that due to the increase in temperature, the physical bonds become weak among CV molecules and the vacant site of the sorbent. These might be a reason that leads to a shrinking in elimination percentage. The solubility of the CV dye augmented amid the rise in temperature. Subsequently, the associated forces among the solute and solvent were vigorous compared to solute and the sorbent, resulting in strenuously in solute adsorbing [32]. The ascertained progression in decreasing the percentage removal efficacy with the rise in temperature revealed that CV sorption onto CaO modified fly ash surface is an exothermic process.
4.4. Adsorption IsothermsEquilibrium models have evaluated in the present work. The models applied in the current investigation were Langmuir, Freundlich, Dubinin-Raduskevich (D-R) to ascertain the equilibrium mechanism. The factors and correlation coefficient determined as of the graph of Langmuir (Ce/qe Vs. Ce), Freundlich (log qe Vs. log Ce), and D-R (ln qe Vs. ɛ2) (not shown in the figure) enlisted in Table 1.
It is manifest from Table 1; the Langmuir equilibrium model showed well fitted with corresponding data accompanied by the highest correlation coefficients at preselected temperatures (not shown in the figure). The higher sorption capacity of modified fly ash was observed 38.57 mg g−1 at 303 K. The calculated value of KL and qm has shown a negative relation with the temperature. An increase in the temperature of the sorption study encourages the reduction of maximum sorption capacity.
Moreover, Freundlich equilibrium model reasonably fit the obtained isothermal data at all preselected temperature (R2 > 0.97). It is evident from the analytical data that KF showed a negative relation to a temperature Table 1, inferred that sorption is an exothermic process. The degree of n suggests the suitability of the sorption process. The calculated value of n lies within the range of 1 and 10 (i.e., 1/n < 1) indicates the relevance of the sorption process. In this study, the obtained results are in agreement with this theory.
To distinguish the physisorption and chemisorption on the heterogeneous surfaces, the isothermal data evaluated by the D-R model. The obtained correlation coefficient value was less compared to the Freundlich and Langmuir equilibrium models (R2 D-R < Freundlich < Langmuir) Table 1. The constant β manifested regarding the mean free energy E (KJ mol−1) of the sorption when 1 mole of the sorbate gets entered onto the solid surfaces from the absolute solution and can be determined to apply the following equation Eq. (6):
By solving the above equation, the mechanism of adsorption inferred. The sorption process proceeds to chemisorption when E ≥ 8 to 16 KJ mol−1 and termed as physical sorption when E < 8 KJ mol−1 [23]. The sorption of CV onto CaO modified fly ash regarded as a chemisorption mechanism revealed from the value of E, i.e., > 8 KJ mol−1.
The experimental data obeyed the Langmuir equilibrium model, suggesting that the adhering energy of the molecule was identical to the whole surface area. Furthermore, it has revealed that the sorbed dye molecules have inert associations with each other and establishing a monolayer.
4.5. Adsorption KineticsFor comprehending the process, kinetics involved in reactions, like Pseudo-First order, Pseudo-Second order, and Intra-particle diffusion models assessed. The data of equilibrium sorption capacity, rate constants, and nature of sorption were investigated using these three models. The rate constant k1 for pseudo-first-order and equilibrium sorption capacity qe evaluate the slope and intercept of the graphs of (qe-qt) Vs. t (not shown in the figure) enlisted in Table 2 amid the R2 (correlation coefficient). It reveals from the present study, Table 2 that the analytical data was not obeying the pseudo-first-order kinetics.
Furthermore, comprehending the kinetics of CV sorption, the pseudo-second-order kinetic model employed using Eq. (8). The rate constant k2 for pseudo-second-order assessed from the slope and intercept of the graphs of t/qt Vs. t. at preselected temperatures deciphered in Fig. 3. Moreover, the R2 for the pseudo-second-order equation exhibited well agreement (R2 > 0.995) compare to the pseudo-first-order correlation coefficient. It suggests from Table 2 that the pseudo-second-order kinetic model is favorable to the present study.
The rate constant k2 showed a negative relation with the temperature affirmed that CV sorption is an exothermic process. The experimental and theoretical values of qe were relatively closer under observed temperatures, revealing that the sorption process obeyed the pseudo-second-order kinetic model. Observations exhibited that CV sorption onto CaO modified fly ash is a chemisorption process. The initial sorption rate, h (mg g−1 min−1) determine by applying Eq. (14):
Table 2 illustrated that the initial sorption rate, h was not suitable for high temperatures as its value decreased at high temperatures. The Weber-Morris model (Eq. (8)) was used to determine the intraparticle diffusion. To comprehend the diffusion Weber-Morris graph (qe Vs. t0.5) drawn. The curve suggests that the primary area resembles the outer surface apprehension; the next step corresponds to the reasonable perception revealing that the intraparticle diffusion was the rate hindrance step. The final step conveyed the isothermal apprehension. The obtained result narrates that the intraparticle diffusion intricate in the present study. Furthermore, it observes that apart from the process as mentioned, earlier other mechanisms might play a significant role.
4.6. Activation Energy and Thermodynamic FactorsArrhenius equation (Eq. (10)) applied to assess the activation energy. The rate constant k2 obtained from the pseudo-second-order Table 2. The graph has been plotted between ln k2 and 1/T Fig. S3. From the slope of the linear plot, Ea achieved. The obtained Ea for the adsorption was – 42.25 KJ mol−1. It affirmed by the activation energy that the chemisorption phenomenon is the prevailing reaction. From the literature, it is manifest that two kinds of sorption are present, i.e., physisorption and chemisorption. Physisorption is weak, and its value is less than 40 KJ mol−1. Chemisorption involves strong forces compare to physisorption and possesses a higher magnitude [22]. Hence, the obtained value of the activation energy reveals that the chemisorption mechanism is evolved for CV sorption on CaO modified fly ash sample surface.
Gibb’s free energy (ΔG°) has been calculated by applying the Eq. (11) for modified fly ash at all preselected temperatures for the sorption of CV from the simulated solution has been provided in Table 3. The entropy (ΔS°) and enthalpy (ΔH°) values have been enlisted Table 3 and evaluated from the slope and intercept of the plot between Gibb’s free energy (ΔG°) and temperature (T) shown in Fig. S4. It is manifest from the literature that the negative obtained value of Gibb’s free energy (ΔG°) suggests the spontaneity of the reaction. The obtained values of Gibb’s free energy for the present study are in agreement with the literature. The increase in the value of Gibb’s free energy (ΔG°) with increasing temperature revealed that sorption proceeds smoothly at low temperatures. The present study showed the exothermic nature of the reaction affirmed by the negative obtained value of enthalpy (ΔH°). It could be assumed the character of sorption via the value of enthalpy (ΔH°). Therefore, CV adsorption by CaO modified fly ash ascribed to the chemisorption phenomena affirmed by negative values of enthalpy. The sorption process of CV on CaO modified fly ash was solely governed by the enthalpy, and it confirms by the entropy’s negative value.
5. ConclusionsIn the present work, CaO, modified fly ash, was considered an efficient sorbent to eradicate CV dye from the simulated solution in a batch study. The role of different parameters influencing the sorption process like primary dye concentration, sorption contact time, the amount of adsorbent, temperature, along the initial pH of the simulated solution has investigated. The comparison of the maximum adsorption capacity of CV dye by different adsorbents with the fly ash used in this study has been shown in Table S3. It is evident from the obtained experimental data that the removal percentage of the CV dye decreased with the increment of the primary dye concentration and subsequently increased with increasing sorption contact time, and the amount of the sorbent up to a specific limit. The higher removal achieved at pH 8.0. It has manifested from the isotherm study that the experimental data obeyed the Langmuir model. The equilibrium study reveals that monolayer adsorption happening on the surface of the CaO modified fly ash sample surface. It concludes from the obtained data that with rising temperature (303–333 K) leads to a shrinking in the monolayer adsorption capacity. The higher value of sorption capacity achieved at 303 K was determined to be 38.57 mg g−1. The Dubinin-Raduskevich (D-R) isotherm reveals the chemisorption nature of CV dye on CaO modified fly ash.
Additionally, the kinetic analysis also inferred the chemisorption phenomenon. The calculated experimental data affirmed that intra-particle diffusion was not the sole rate-limiting factor. Based on the Arrhenius equation, the calculation was done for the determination of the activation energy (Ea) of the sorption process. The obtained value of Ea 42.25 KJ mol−1 showed that the nature of CV sorption on the CaO modified fly ash surface is chemisorption. Gibbs free energy (ΔG°), Enthalpy (ΔH°), along with Entropy (ΔS°) have assessed. The acquired thermodynamic data portrays the spontaneous and exothermic nature of the sorption. Furthermore, it suggests that CaO modified fly ash could be a cheap and promising adsorbent anticipated for the eradication of the CV dye from the simulated solution along with the industrial wastewater.
NomenclatureCi Initial dye concentration (mg L−1) h Initial adsorption rate (mg g−1 min−1) I Intraparticle diffusion model constant KC Distribution coefficient for biosorption KL Langmuir constant (L mg−1) ki Intraparticle diffusion rate constant (mg g−1min−0.5) k1 Pseudo-first-order rate constant (min−1) k2 Pseudo-second-order rate constant (g mg−1 min−1) m mass of biosorbent (g) n Freundlich adsorption isotherm constant qe Equilibrium dye concentration on biosorbent (mg g−1) qm Maximum biosorption capacity (mg g−1) qexp Maximum sorption capacity experimental (mg g−1) qt Amount of dye adsorbed at time t (mg g−1) R Universal gas constant (8.314 J mol−1K−1) V The volume of the solution (L) AcknowledgmentsThis study was funded by the National Natural Science Foundation of China 21850410446.
NotesAuthor Contributions S.C. (Senior Researcher) designed the experiments, collected the data and jointly wrote the paper. A.M. (B.Tech Student) did literature survey, revised the manuscript. S.D. (Research Engineer) did data compilation and interpretation of the experimental work. N.R.M. (Professor) reviewed the whole work and provided suggestions for the improvement of the paper. S.I. (Ph.D student) jointly discussed, analyzed, and wrote the paper. P.D. (Professor) supervised and mentored the overall work. References1. Choi HJ, Yu SW. Biosorption of methylene blue from aqueous solution by agricultural bioadsorbent corncob. Environ Eng Res. 2019;24:99–106.
2. Ayadi I, Souissi Y, Jlassi I, Peixoto F, Mnif W. Chemical synonyms, molecular structure and toxicological risk assessment of synthetic textile dyes: A critical review. J Dev Drugs. 2016;5:1–4.
3. Paz A, Carballo J, Perez MJ, Dominguez JM. Biological treatment of model dyes and textile wastewaters. Chemosphere. 2017;181:168–177.
4. Adeyemo AA, Adeoye IO, Bello OS. Adsorption of dyes using different types of clay: A review. Appl Water Sci. 2017;7:543–568.
5. Hema N, Satish S, Suresha S. Decolorization of a synthetic dye-yellow 5gn by pseudomonas aeruginosa using response surface methodology. J Microbiol Biotech Res. 2017;3:147–154.
6. Velmurugan S, Ganesh B, Babuponnusami A, Rajasekaran R. Decolourisation of reactive blue 28 from dye wastewater by photo-Fenton process and sono-Fenton processes. Int J Chem Sci. 2016;14:1433–1446.
7. Liu Y, Jin W, Zhao Y, Zhang G, Zhang W. Enhanced catalytic degradation of methylene blue by Fe2O3/GO via heterogeneous photo-Fenton reactions. Appl Catal B: Environ. 2017;206:642–652.
8. Reza KM, Kurny A, Gulshan F. Parameters affecting the photo-catalytic degradation of dyes using TiO2: A review. Appl Water Sci. 2017;7:1569–1578.
9. Azad A, Rasul MG, Mofijur M, Bhuiya MK, Mondal SK, Sattar MK. Energy and waste management for petroleum refining effluents: A case study in Bangladesh. Int J Automot Mech Eng. 2015;2170–2186.
10. Kumar M, Puri A. A review of permissible limits of drinking water. Indian J Occup Environ Med. 2012;16:40–44.
12. Kar S, Das RN, Roy K, Leszczynski J. Can toxicity for different species be correlated? The concept and emerging applications of interspecies quantitative structure-toxicity relationship (i-qstr) modeling. IJQSPR. 2016;1:23–51.
13. Kazandjieva J, Tsankov N. Tattoos: Dermatological complications. Clin Dermatol. 2007;25:375–382.
14. Sainio EL, Jolanki R, Hakala E, Kanerva L. Metals and arsenic in eye shadows. Contact Dermatitis. 2000;42:5–10.
15. Vakili M, Rafatullah M, Salamatinia B, et al. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr Polym. 2014;113:115–130.
16. Filho ACD, Mazzocco AC, Dotto GL, Thue PS, Pavan FA. Eragrostis plana nees as a novel eco-friendly adsorbent for removal of crystal violet from aqueous solution. Environ Sci Pollut Res Int. 2017;24:19909–19919.
17. Martins LR, Rodrigues JAV, Adarme OFH, Melo TMS, Gurgel LVA, Gil LF. Optimization of cellulose and sugarcane bagasse oxidation: Application for adsorptive removal of crystal violet and auramine-o from aqueous solution. J Colloid Interface Sci. 2017;494:223–241.
18. Arumugham T, Amimodu GR, Kaleekkal NJ. Nano CuO/g-C3N4 sheets-based ultrafiltration membrane with enhanced interfacial affinity, antifouling and protein separation performances for water treatment application. J Environ Sci. 2019;82:57–69.
19. Cinperi NC, Ozturk E, Yigit NO, Kitis M. Treatment of woolen textile wastewater using membrane bioreactor, nanofiltration and reverse osmosis for reuse in production processes. J Clean Prod. 2019;223:837–848.
20. Sahinkaya E, Tuncman S, Koc I, et al. Performance of a pilot-scale reverse osmosis process for water recovery from biologically-treated textile wastewater. J Environ Manage. 2019;249:09382.
21. Li L, Zheng X, Chi Y, et al. Molecularly imprinted carbon nanosheets supported TiO2: Strong selectivity and synergic adsorption-photocatalysis for antibiotics removal. J Hazard Mater. 2020;383:121211.
22. Singh A, Sonal S, Kumar R, Mishra BK. Adsorption of chlorhexidine digluconate on acid-modified fly ash: Kinetics, isotherms and influencing factors. Environ Eng Res. 2019;25:205–211.
23. Farooq S, Saeed A, Sharif M, Hussain J, Mabood F, Iftekhar M. Process optimization studies of crystal violet dye adsorption onto novel, mixed metal Ni0.5Co0.5Fe2O4 ferrospinel nanoparticles using factorial design. J Water Process Eng. 2017;16:132–141.
24. Senthilkumar S, Kalaamani P, Subburaam C. Liquid phase adsorption of crystal violet onto activated carbons derived from male flowers of coconut tree. J Hazard Mater. 2006;136:800–808.
25. Veetil NP, Gandhimathi R, Thanga RS, Anantha Singh TS. Adsorption and desorption characteristics of crystal violet in bottom ash column. J Urban Environ Eng. 2012;6:18–29.
26. Niveditha SV, Gandhimathi R. Flyash augmented Fe3O4 as a heterogeneous catalyst for degradation of stabilized landfill leachate in Fenton process. Chemosphere. 2020;242:125189.
27. Wang S, Boyjoo Y, Choueib A, Zhu Z. Removal of dyes from aqueous solution using fly ash and red mud. Water Res. 2005;9:129–138.
28. Temuujin JV, Van Riessen A, Williams R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J Hazard Mater. 2009;167:82–88.
29. Patel A. Investigation of partial CO2 capture using ash from CFB boilers with limestone injection. [dissertation]. Halifax: Dalhousie University; 2016.
30. Saha PD, Chakraborty S, Das S. Optimization of hazardous crystal violet by chemically treated rice husk: Using central composite design response surface methodology. Arch Environ Sci. 2012;6:57–61.
31. Chakraborty S, Chowdhury S, Saha PD. Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym. 2011;86:1533–1541.
32. Chowdhury S, Chakraborty S, Das P. Adsorption of crystal violet from aqueous solution by citric acid modified rice straw: equilibrium, kinetics, and thermodynamics. Sep Sci Technol. 2013;48:1339–1348.
33. Sahoo PK, Tripathy S, Panigrahi MK, Equeenuddin SM. Evaluation of the use of an alkali modified fly ash as a potential adsorbent for the removal of metals from acid mine drainage. Appl Water Sci. 2013;3:567–576.
34. Visa M, Chelaru AM. Hydrothermally modified fly ash for heavy metals and dyes removal in advanced wastewater treatment. Appl Water Sci. 2014;303:14–22.
35. Gemini BT, Ozel HU, Ozel HB. Adsorption behaviors of crystal violet from aqueous solution using Anatolian black pine (Pinus nigra Arnold.): kinetic and equilibrium studies. Sep Sci Technol. 2019;55:406–414.
36. Chakraborty S, Chowdhury S, Das SP. Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk. Carbohydr Polym. 2011;86:1533–1541.
37. Saha PD, Chakraborty S, Chowdhury S. Batch and continuous (fixed-bed column) biosorption of crystal violet by Artocarpus heterophyllus (jackfruit) leaf powder. Colloid Surface B. 2012;92:262–270.
38. Chakraborty S, Chowdhury S, Saha PD. Biosorption of hazardous textile dyes from aqueous solutions by hen feathers: Batch and column studies. Korean J Chem Eng. 2012;29:1567–1576.
39. Ahmad R. Studies on adsorption of crystal violet dye from aqueous solution onto coniferous pinus bark powder (CPBP). J Hazard Mater. 2009;171:767–773.
40. Ahmed M, Mashkoor F, Nasar A. Development, characterization, and utilization of magnetized orange peel waste as a novel adsorbent for the confiscation of crystal violet dye from aqueous solution. Groundw Sustain Dev. 2019;10:100322.
41. Annadurai G, Juang RS, Lee DJ. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J Hazard Mater. 2014;92:263–274.
42. Cheruiyot GK, Wanyonyi WC, Kiplimo JJ, Maina EN. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci African. 2019;5:e00116.
Fig. 2Effect of different factors on the adsorption of crystal violet by unmodified and calcium oxide modified fly ash (a) Effect of pH, (b) Effect of amount of adsorbent, (c) Effect of primary dye concentration, and (d) Effect of temperature. 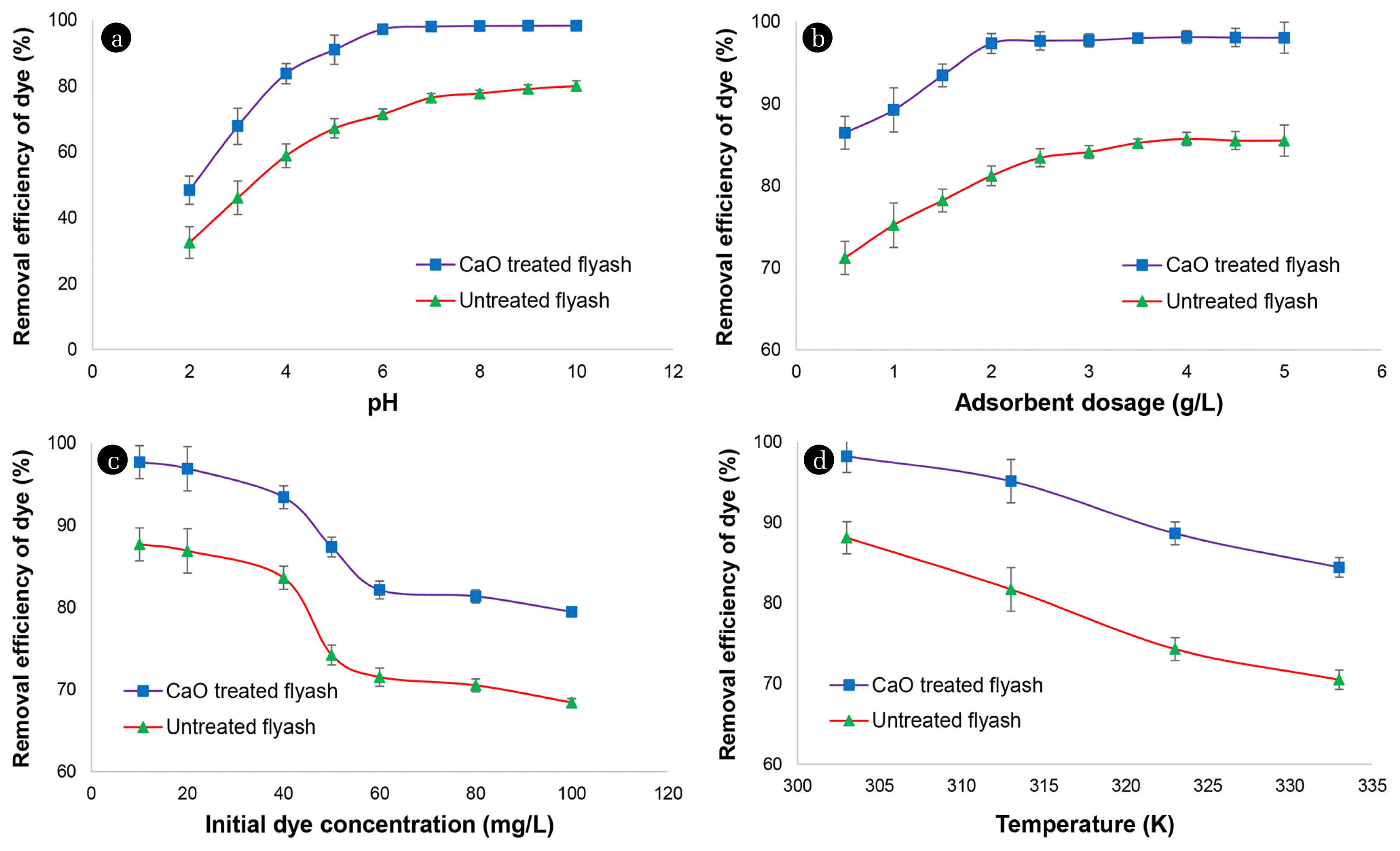
Fig. 3Pseudo-second order kinetic plots for adsorption of crystal violet by calcium oxide modified fly ash. (experimental conditions: primary dye concentration: 50 mg L−1, amount of adsorbent: 2 g L−1, agitation speed: 140 r/min, pH of primary solution: 8.0 temperature: 303K.) 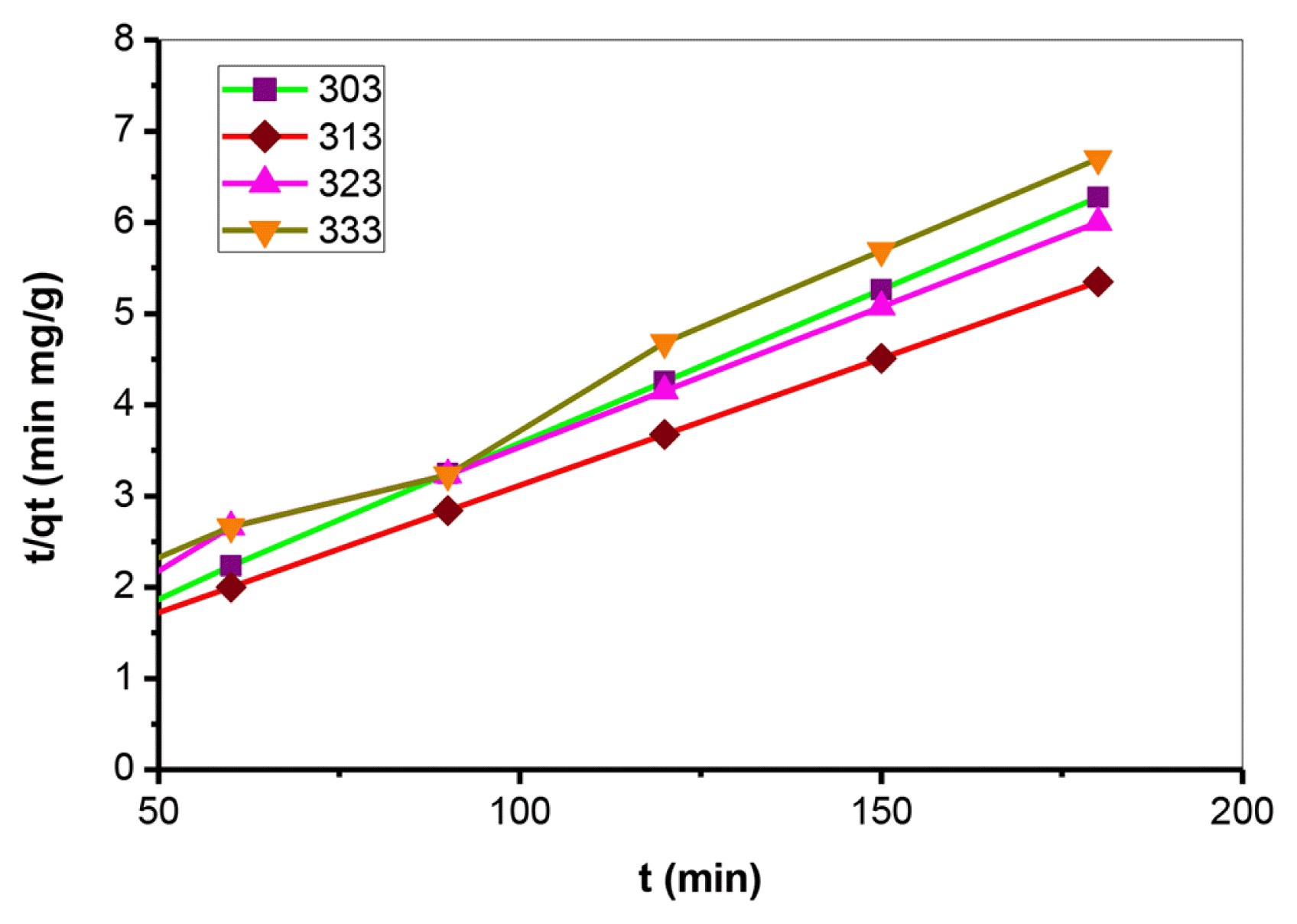
Table 1Adsorption Isotherm Constants for Adsorption of Crystal Violet onto Calcium Oxide Modified Fly Ash at Preselected Temperatures Table 2Kinetic Parameters for Adsorption of Crystal Violet onto Calcium Oxide Modified Fly Ash |
|
||||||||||||||||||||||||||||||||||||||||