1. Introduction
Arsenic, one of the toxic metallic contaminants, can be found in natural sulfidic minerals or sediments [1]. Arsenic is generally released into the water by global geochemical activities [2]. However, human activities such as mining, burning fossil fuels [1], and smelting [3] also release anthropogenic arsenic compounds. Arsenic contamination in water sources could affect water-related activities such as rice cultivation [4]. Main species of arsenic in groundwater are the inorganic form, as pentavalent under the aerobic condition and trivalent under anaerobic condition [5]. Arsenic in drinking water is one of the well-known carcinogens, especially trivalent arsenic was reported to be greater toxicity than pentavalent arsenic [1]. Oral ingestion of pentavalent arsenic also reduced into trivalent arsenic in the human cell causing various skin diseases including cancer [6].Therefore, the World Health Organization (WHO) has determined drinking water quality criteria to be 0.01mg-As/L or less because aqueous arsenic adversely affects human health [7].
To remove the excessive arsenic from water sources, oxidation [8], flocculation and filtration [9], coprecipitation [10], and adsorption methods have been reported. Among these methods, adsorption needs simple operation process and does not create sludge as a waste [1]. Activated alumina [11], iron-coated adsorbents [12], ion exchange resin [13], and activated carbon [14] can be utilized as an adsorbent. Activated carbon is usually prepared from waste such as rice husks [11], coconut husks [15], and petroleum cokes [16], and the production costs would be cheaper than other adsorbents. In addition to the carbonaceous materials have stronger resistance heat and acid-resistant than other polymeric ion exchangers have, therefore activated carbon is more flexible than other adsorbents. The activated carbon is widely used for the adsorption of volatile organic compounds (VOCs) because of its porous structure and large specific surface area [17].
In our previous studies, heating and nitrogen-doping treatments increased the anion adsorption capacity of activated carbons. Heating activated carbon at 950°C for enhancing the π-electrons on the graphene layer [18] and forming quaternary nitrogen from pyridine type nitrogen by thermal decomposition were conducted [19]. We also conducted thermal chemical vapor deposition (CVD) for doping nitrogen onto the carbon surface [20]. Combination of the CVD, steam activation and thermal outgassing successfully enhanced the anion adsorption capacity [21]. In these studies, we used commercial activated carbon fibers (ACFs) which have 1.5 wt% of nitrogen content for CVD treatment as a raw material. However, it was difficult to distinguish the nitrogen already present in the commercial ACFs from that introduced by the CVD process, in which both played an important role for enhancing anion adsorption capacity. Therefore, we prepared nitrogen-doped material using nitrogen-free phenol resin as a raw material to determine whether the introduced nitrogen could act as adsorption sites for anion adsorption in this study. Furthermore, we also determined the effective procedure for CVD treatment via comparison of surface properties and arsenate adsorption capacities. Because we demonstrated the combination of the CVD, steam activation, and thermal outgassing treatment to enhance the anion adsorption capacity, the optimal procedure has not been clarified in our previous study.
2. Materials and Methods
2.1. Reagents
All the reagents used in this study were analytical grade and used without purification. Phenol resin (Kynol Fiber KF-0270) was purchased from GunEi Chemical Industry Co., Ltd., Japan. The manufacturer describes this phenol resin as a novoloid fiber, and its carbon, hydrogen, nitrogen and oxygen conent are 76, 6, 0 and 18 wt%, respectively. Phenol resin was dried at 110°C for 30 min before conducting treatments.
2.2. Adsorbent Preparation
All treatments were performed in a tubular reactor. Quartz tube was used as a reactor and it was 28 mm outer diameter × 24 mm inner diameter × 0.60 m length in size. All experimental methods were performed under a helium flow, and the initial amount of the phenol resin was 6 g. Under these experimental conditions, we conducted heat treatment, steam activation, and thermal chemical vapor deposition for the phenol resin consecutively. For the heat treatment, samples were heated at 950°C for 30 min. The main purpose of heat treatment is removing oxygen from the surface of ACs and quaternarization of surficial nitrogen. The samples treated in these processes were named as 9.5HT30. In steam activation step, 30 or 50 mL of distilled water was introduced at 800°C. The flow rate was 0.5 mL/min. The samples treated in these processes were named as 8ST30 and 8ST50, respectively. Thermal chemical vapor deposition (CVD) was performed using aniline. The amount of the introduced aniline was 10 mL, and the flow rate and temperature were also 0.5 mL/min as well as the steam activation. The samples modified using thermal CVD process were named as 8ANL10. After the CVD process, the newly formed structure plugs the pore of activated carbon. Since the porous structure of AC is an important parameter of the adsorption capacity, we conducted steam activation after CVD as a post treatment to raise the specific surface area. All prepared samples prepared in this study were listed in Table 1. We numbered each prepared sample and hereafter used these numbers for description.
2.3. Adsorption Studies
The arsenate adsorption experiments were performed using NaH2AsO4·7H2O aqueous solution. The initial concentration of arsenate was varied from 5 to 100 mg-As(V)/L. In 30 mL Erlenmeyer flask, 30 mg of prepared samples and 15 mL arsenate solution were added. The solution pH was measured with a pH meter (D-51, Horiba). Moreover, hydrochloric acid and sodium hydroxide were added to adjust the solution pH. At the room temperature, the mixtures were agitated at 100 rpm for 24 h to reach the equilibrium state. Arsenate concentration was determined by ion chromatograph (ICS-1100, Nippon Dionex KK), and adsorption amount at the equilibrium state was calculated by the following equation:
where C0 and Ce represent the initial and the equilibrium arsenate concentrations [mmol/L], respectively. v represents solution volume [mL] and w indicates the weight of adsorbent [mg].
To determine the optimal adsorption time, adsorption kinetics in aqueous condition were examined. One hundred mg of AC samples were added into 200 mL of 100 mg-As/L solution. The mixture was agitated at 100 rpm at room temperature, and residual concentration of arsenate at a certain time was sampled and examined. All experimental data were fitted to the following Eq. (2) and (3), which represent the pseudo-first-order and the pseudo-second- order kinetic model, respectively.
where Qt and Qe [mmol/L] represent the amount of adsorption at the certain time and equilibrium state, respectively. k1 [1/h] is pseudo-first order rate constant, and k2 [g/mmol h] is pseudo-second order rate constant.
The obtained experimental adsorption data were fitted to the Langmuir and Freundlich isotherm models. We applied the following equation as a Langmuir isotherm model.
where Ce represents the arsenate concentration at equilibrium state. KL [L/mmol] refers to the Langmuir isotherm constant. Qe and Qm [mmol/L] are the adsorption capacity at the equilibrium and maximum adsorption capacity, respectively. Freundlich isotherm model can be written as the following equation:
where KF [(mmol/g) (L/mmol)1/n] is the Freundlich isotherm constant, and 1/n is related to the heterogeneity of the surface [22].
2.4. Adsorbent Properties
Pore characteristics of the samples were determined by BET measurement using N2 adsorption/desorption isotherms at liquid N2 temperature (BELSORP-mini II, Microtrac BEL). Prepared samples were outgassed at 300°C for an hour before the analysis. Using this method, the specific surface area (SBET) and average pore size (Davg) of the samples were calculated. The total pore volume (Vtotal) was calculated from the maximum nitrogen adsorption capacity at a relative pressure of 0.990. The micropore volume (Vmicro) was calculated by SPE method using αs plots [23].
Elemental composition of the prepared samples was determined by CHN elemental analyzer (PE2400II, Perkin Elmer). We assumed that the prepared samples are composed of carbon, hydrogen, nitrogen and oxygen. Oxygen content was calculated by balance.
Boehm titration method was used to determine the amount of surface basic functional groups on the prepared activated carbon samples. Solutions of 0.1 mol/L NaOH and 0.1 mol/L HCl were prepared, and 25 mL of each solution and 200 mg of each sample were added in a 100 mL Erlenmeyer flask. The flasks were agitated for 4 d at 25°C. After the shaking process, 5 mL of each solution was sampled and titrated with 0.05 mL HCl. In the case of the 0.1 mol/L HCl, 10 mL of 0.1 mol/L NaOH was added to perform back titration.
3. Results and Discussion
3.1. Adsorption Kinetics of Arsenate
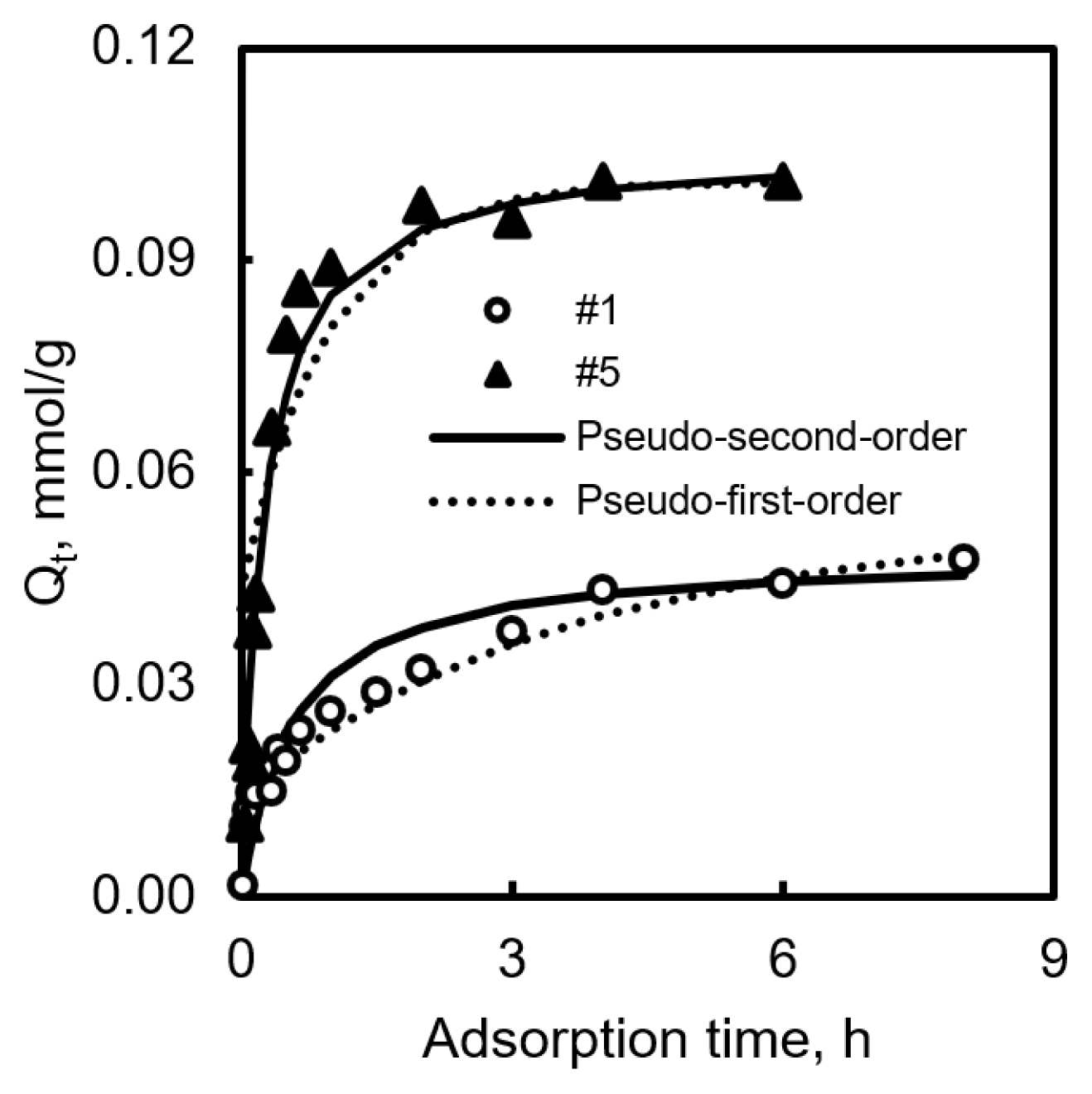
To compare the kinetic properties between non-CVD and CVD-treated samples, we choose samples #1 and #5. Fig. 1 and Table 2 display the results. Sample #1, which was prepared by steam activation and heat treatment, and its experimental results, followed the pseudo-second-order equation. It required 4 h to reach the equilibrium state. In the case of sample #5, it also followed the pseudo-second-order equation. Pseudo-first order model suggests that the rate-limiting step could be considered as a collision between adsorbent and adsorbate, and pseudo-second order model represents pore diffusion. CVD-treated samples also required 4 h to reach the equilibrium state, because of its high rate constant (k2), and the amount of adsorption increased faster in the first 2 h than non-CVD samples [24].
3.2. Effect of pH on the Arsenate Adsorption on the Activated Carbons
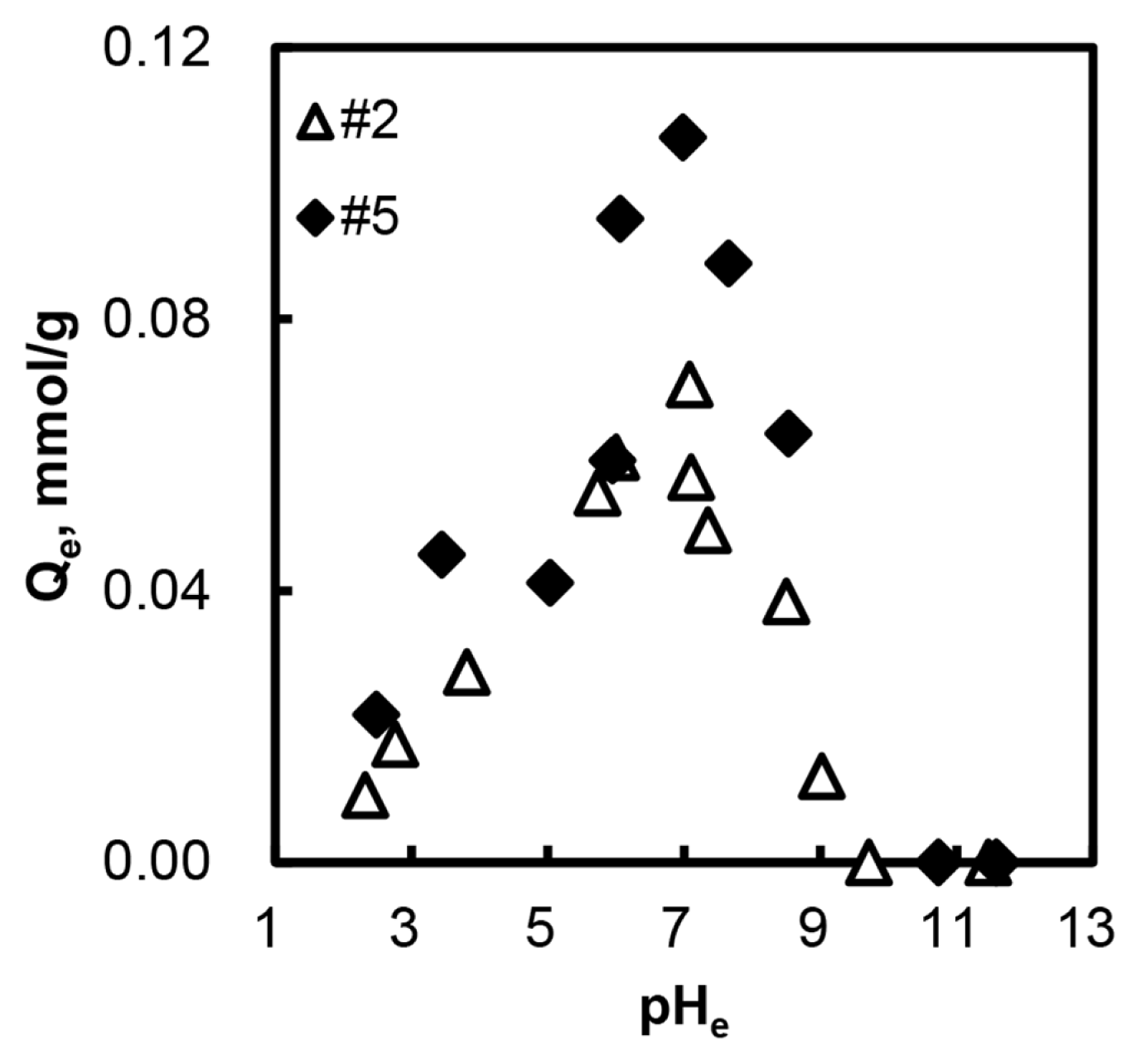
Fig. 2 describes the relationship between the equilibrium pH and arsenate adsorption capacity. The results pointed out that the pHe 7 was the best pH condition for arsenate adsorption. We adjusted the solution pH for arsenic adsorption to 6.8–7.2 in further experiments. When the pHe was greater than 11, the adsorption capacities reached zero, whereas the adsorption capacity did not decrease to zero at pHe 3. The pKa of H3AsO4 is 2.19, 6.94, and 11.5. When the pHe is 11, HAsO4 2− and AsO43− are the main species in aqueous solution. At pH 3, the dominant species of arsenate are H3AsO4 and H2AsO4 −. In our preliminary study [14], anion adsorption mechanism was based on the electrostatic attraction and ion exchange between adsorbent and adsorbate. Therefore, when the pH is lower than 4, the neutral form of arsenate exists in the solution, and it could not be effectively adsorbed onto the adsorbent. Moreover, we added hydrochloric acid for adjusting solution pH, and Cl− would be a competitive anion with arsenate. On the other hand, when the solution pH is higher than 4, the dominant species of arsenate are anion form; therefore the anionic arsenate would be appropriate for adsorption. As the experimental results point out, the adsorption capacity was increased when the solution pH rose up to 7. If the solution pH is greater than 7, solution OHconcentration also increases. Kwok [25] reported that the excessive OH− showed a negative effect on arsenic adsorption because the ion exchange occurred between OH− and anionic arsenates. Moreover, the repulsive interaction between the negatively charged arsenic ion and negatively charged AC surface occurs at the basic pH range. Hence, the adsorption capacity decreased when the pHe increased at the pH range 7–11. Comparing these two competitive ions, OH− would have stronger negative effects on arsenate adsorption. In conclusion, when the pHe is lower than 7, the adsorption capacity strongly depended on the concentration of anionic arsenate form. However, the hydroxide concentration was an important factor when the pHe is bigger than 7.
3.3. Adsorption Isotherm
Table 1 describes the adsorption capacity of the prepared samples for arsenate adsorption. The adsorption isotherms for prepared samples are shown in Fig. 2. We choose the best samples from non-CVD samples and CVD-treat samples to depict isotherm. Table 3 shows the calculated Langmuir and Freundlich parameters. Coefficient of determination R2 indicated that the Langmuir isotherm was the best fitting.
3.4. Adsorbent Properties
Porous structural parameters of prepared samples are shown in Table 4. Samples #1, #2 and #6 pointed out that the specific surface area depended on the amount of the introduced distilled water. Sample #7 indicated SBET of only 4 m2/g, implying that aniline CVD process successfully formed a new surface on the precursor. Incidentally, the order of heat treatment and steam activation could affect the surficial property; therefore we also prepared samples, which were sample #4 and #5. Sample #4 had SBET of 936 m2/g, which was only 71% of sample #5. From this result, heat treatment enhances the robustness against the activation process, and therefore heat treatment should be conducted finally for the effective activation process. Qe/SBET was also calculated for comparing adsorption effectiveness. CVD-treated samples (samples #3–#5) showed at least 18% higher Qe/SBET value than that for non-CVD treated samples (samples #1 and #2). In the case of sample #8, steam activation was not conducted as a pretreatment, showing that nitrogen doping would not be conducted properly.
Table 5 shows the elemental composition of the prepared samples. Nitrogen contents of samples #1 and #2, which were not treated with aniline, were 0.24 wt% and 0.27 wt%, respectively, while those of all CVD-treated samples (#3–#5, #8) were at least 37% greater than those of samples #1 and #2. In the case of sample #3, the specific surface area was the lowest among the CVD-treated samples, and nitrogen content was 53% of sample #4. These results exhibited that the heat treatment as a pre-treatment for CVD was not appropriate. Containing high specific surface area is important for precursors of the CVD process because the purpose of steam activation as a pre-treatment is to increase the contact area between the chemical vapor and the sample surface. Nitrogen content of sample #5 and #8 demonstrated this idea, because sample #5 showed 41% higher nitrogen content than sample #8, and the difference between samples #5 and #8 is only the steam activation as a pre-treatment. Therefore, the high specific surface area is an important factor for the precursor in the CVD process. In the same way, the newly formed nitrogen-rich surface by aniline would become hard to be activated using the steam after the heat treatment. Sample #4 showed the highest nitrogen content, but the specific surface area was 71% of sample #5 because of that reason. To sum up, 8ST30-8ANL10-8ST50-9.5HT30 (sample #5) was the most effective order for preparing thermal CVD-treated activated carbon from phenol resin as a raw material.
Total basic functional groups of steam-treated samples (samples #1–#5) were shown in Table 5. In our previous study, the high amount of basic functional groups on the activated carbon was the most important parameter for effective arsenate adsorption [14]. As examples of the basic functional groups, Ota et al. [18] reported that π-electrons on the graphene layer can attract some protons resulting in a slight increase of adsorption site. This adsorption mechanism was also observed in this study. The initial solution pH was 6.94 at the initial arsenate concentration of 100 mg-As(V)/L, and the equilibrium pH for samples #2 and #5 was 7.21 and 7.29, respectively. Moreover, pyridinic nitrogen (N-6), pyrrolic nitrogen (N-5) and amine groups could become positively charged adsorption sites below certain pH which depends on their pKa, whereas the quaternary nitrogen (N-Q) always exists as positively charged sites [20, 26]. Furthermore, oxygen-containing basic groups generated by thermal decomposition of oxygenated acidic groups could act as basic sites [27]. Hence, these potential adsorption sites could be measured by Boehm titration except N-Q. But the Boehm titration is using 0.1 M HCl to neutralize the basic functional groups. Therefore, Boehm titration is hard to differentiate among weak and strong nitrogen-containing basic functional groups, oxygen- containing basic surface functional groups and π-electrons on the graphene layer [27]. The number of basic functional groups increased between samples #1 and #2, and adsorption capacity also increased when the specific surface area increased. It could be assumed that large specific surface area accommodated more protons and it works as adsorption sites for arsenate. At the acidic pHe range of 2–6, the adsorption capacity of CVD-treated samples would be higher than non-CVD samples because N-5 and N-6 might be formed by the CVD process. Also, in the case of sample #5, it showed the highest oxygen content than other samples. It means that sample #5 has the highest oxygen-containing acidic or basic functional groups. However, there was no significant difference in adsorption capacity for samples #2 at the acidic pH range. Oxygen-containing acidic functional groups are known as electron withdrawing groups, and excessive acidic oxygenated functional groups diminish the π-electrons on the graphene layer. Sample #5 showed the lower number of basic functional group than that of non-CVD samples, but it showed the highest oxygen content. In conclusion, the oxygen in the CVD-treated samples could mainly exist as oxygen-containing acidic functional groups. This result also explains that sample #3 showed the lowest basic functional groups, but their oxygen content was nearly the same as non-CVD samples.
3.5. Comparisons of Arsenic Adsorption Capacities of Various Adsorbents
Comparisons of arsenic(V) adsorption capacities of the various adsorbents are shown in Table 6. In this study, we prepared activated carbon derived from phenol resin and showed better adsorption capacity than that of our previous study [14]. Moreover, we also showed that the amount of basic functional groups was not always related to arsenic adsorption at pH 7 which was optimal pH condition for arsenic adsorption. In addition, if the precursor is nitrogen-rich and that nitrogen has robustness against heat, it is expected to enhance the arsenic adsorption capacity. In a recent study, activated carbons which were loaded with other materials on the surface such as iron particles are mainly used for arsenic(V) removal [29, 31]. Zhang et al. [29] stated that the activated carbon fibers were better supporting matrix for iron oxides. We used fiber-type phenol resin as a precursor. Furthermore, non-CVD and CVD-treated samples were followed by the Langmuir isotherm model and pseudo-second order kinetic model. This result reveals that CVD processed samples maintain the properties of adsorbent as activated carbon fiber. Therefore, by using various ACFs containing nitrogen as precursors and using the optimized treatment method (sample #5) which is demonstrated in this study, it is expected that the adsorption capacity can be enhanced through these additional surface modifications.
4. Conclusions
In this study, we conducted heat treatment, steam activation, and thermal CVD to prepare nitrogen-doped AC, and examined the adsorption capacity of the samples to elucidate the most effective preparation order. 8ST30-8ANL10-8ST50-9.5HT30 (sample #5) was the optimal procedure for aniline CVD to prepare adsorbent for arsenic adsorption. Sample #5 showed 0.112 mmol/g of arsenate adsorption capacity, and it was at least 70% higher than that of any other prepared samples. The surface properties which played the most decisive role in the maximum arsenic adsorption amount in this study were the specific surface area and the nitrogen content. Qe/SBET value of CVD-treated samples represented that the newly formed nitrogen on the surface would enhance adsorption capacity. In addition, both non-CVD and CVD-treated samples maintained their adsorption mechanisms between activated carbon fibers and arsenic. To sum up, by using nitrogen-rich ACFs as precursors and conducting the optimized treatment method (sample #5), it is expected that the adsorption capacity can be enhanced through additional surface modifications.