1. Introduction
The sustainable handling of end-of-life lithium-ion batteries (EoL-LiBs) is an issue of global concern [1, 2]. The estimated consumption of LiBs in Pakistan alone is approximately 7 million units that pose a big challenge for current waste disposal strategies [3, 4]. Entry of the EoL-LiBs into solid municipal waste is a potential risk to biota [5–8]. Landfill disposal can result in soil contamination and pollution of surface and groundwater by slow leach process [9–12]. The heavy metal components such as cobalt (Co) and nickel (Ni) are group 2B carcinogen, while lithium (Li) is toxic above 1.2 meq/L in blood [13–15]. Li, as Li0, may cause explosive fires when in contact with water; incineration of EoL-LiBs may result in emission of toxic substances such as furans by degradation of separator and encapsulation membranes. Given that the recycling of EoL-LiBs and recovery of metal values for reuse offers a sustainable technology. Notably, the metals remain in cathode material are potentially high-value rare metals (other than the rare earth elements) as per their abundance, occurrence in natural resource, and criticality for ensuring the green technology [16, 17]. Depending on the type of battery, EoL-LiBs typically contain Co (5–20%), Ni (5–10%), manganese (Mn) (5–15%), and Li (5–7%) [18, 19]. Therefore, the recycling of EoL-LiBs not only offers a benefit to the environment but may also provide an economic benefit by recovery of rare metals to meet the rising demand for these resources [4].
A review of the available literature supports the efficacy of hydrometallurgical processing over the pyro and bio metallurgical recycling strategies [18–21]. The hydrometallurgical processes usually consist of a leaching step for solubilizing the metals into either mineral acid solutions (HCl, H2SO4, H3PO4, HNO3) or organic acid solutions (C2H2O4, C6H8O7, C4H6O4, C6H8O6, C4H6O5), the leach solution may also include a reductant (H2SO3, NH2OH, H2O2) [22–32]. Pregnant leach liquor is treated by various separation and purification steps to recover the metal values [18, 21]. Although there are numerous literature reports concerned with recycling LiCoO2 cathode materials, there are scanty studies for LiNixCoyMnzO2 materials [33, 34]. However, the LiNixCoyMnzO2 cathode material is in widespread usage, hence, a systematic study for recycling the LiNixCoyMnzO2 cathode material will be useful in the circular economy of rare metals.
In the present study, sulfuric acid leaching of the cathode powder has been investigated with and without the addition of the reducing agent (H2O2). The variables like acid concentration, H2O2 dosage, temperature, and leaching time were optimized; the resultant pregnant leach liquor was processed for separation and recovery of rare metals by precipitation and solvent extraction techniques. The novelty of the present work can be considered in its simple operation with a high yield of rare metals as a high purity value- added product. For example, typically the separation of Ni and Co is attained by solvent extraction using Cyanex 272; this requires a strict pH profile to be followed to achieve separation with reasonable efficiency [18, 19, 21]. A change in pH profile will result in poor separation and a Co-loaded organic phase that often needs scrubbing to remove co-extracted Ni. In this study, Ni is selectively precipitated from the leach liquor by complexation with dimethylglyoxime (DMG) prior to recovery of Co by solvent extraction; this eliminates co-extraction possibilities of Ni with Co and the need for a scrubbing step.
2. Experimental
2.1. Materials
A stock sample of LiNixCoyMnzO2 was prepared by collection and homogenization of cathode powder from approximately 50 EoL-LiBs. A wet-chemical analysis of the stock sample by aqua-regia dissolution and ICP analysis found the metal composition to be: Li 7.6%, Co 20.4%, Mn 19.4%, and Ni 19.3%. Acid solutions for the leach studies were prepared by diluting H2SO4 (Lab-Scan Analytical Science, 98% purity) in distilled water. Hydrogen peroxide (H2O2, Sigma Aldrich, 30% purity) was used as the reductant to leach solution when required. Potassium permanganate (KMnO4, 97% purity), DMG (CH3C(NOH)C(NOH)CH3, 99% purity), and sodium carbonate (Na2CO3, 99.9% purity), all supplied by Sigma Aldrich, were used as precipitants for selective precipitation of Mn, Ni, and Li, respectively. A 4.0 M NaOH solution was prepared by addition of pellets (Riedel-deHaën and Lab Scan) to distilled water, this solution was used for pH adjustments and to prepare the Na-Cyanex 272 salt. The Cyanex 272 (85% purity, Sp. gr. 0.92 supplied by Cytec, Canada) was used for solvent extraction of Co. Mineral phase were characterized by X-ray diffraction (XRD; Bruker, D8 Advance diffractometer) at a scan speed of 5 degree/min, Cu Kα radiation 1.5418 Å, generator voltage 40 kV, and 30 mA current.
2.2. Leaching of Cathode Powder
All leach tests were performed as triplicates in 500 mL flat bottom flask. The flask was fitted with a condenser to minimize evaporation, magnetic stirrer bar, and temperature sensor with regulated heating mantle. A 20 g sample of the stock cathode powder was introduced into 400 mL H2SO4 solution of the desired acid concentration; this formed a fixed pulp density of 5% w/v. The solutions were preheated to 90 ± 2°C, unless specified for temperature variation tests. Stirring was maintained at a constant value of 400 rpm. When required, a measured volume of H2O2 in the range of 0–4 vol.% of total leach solution was added as a reducing agent. Progress of the leach reaction was determined by withdrawing sample aliquots at time intervals and immediately filtered using Whatman40 filter paper. The samples were analyzed for the concentration of the metals of interest by ICP (Bruker Varian 820-MS). Leach efficacy was calculated as:
2.3. Precipitation of Metal Compounds
A stock leach liquor generated at the optimized leaching condition, (containing 3.7 g/L Li, 9.6 g/L Ni, 10.1 g/L Co, and 9.6 g/L Mn) was used to investigate metal recovery. Precipitation tests for metals recovery were performed as triplicates. A 200 mL aliquot of leach liquor was placed in a 250 mL glass reactor under the constant stirring speed of 300 rpm. At a first step, the leach liquor was heated to 80°C and pH adjusted to 2.5 by the slow addition of 4.0 M NaOH solution, thereafter, KMnO4 was added under stirring to initiate Mn precipitation. After filtration through Buckner funnel, the filtrate pH was increased to 5.0 at 80°C and then DMG was added under stirring to precipitate the Ni-DMG complex which was subsequently removed filtration using Buckner funnel. The resultant filtrate was then treated by solvent extraction to recover Co (as described in section 2.4). After Co extraction the acidic raffinate was neutralized to pH 7.0 by NaOH addition and heated to 90°C before addition of Na2CO3 to initiate Li precipitation. After the addition of a known quantity of Na2CO3, the alkalinity of solution was raised to 12.0 and maintained at this vale for 60 min. All precipitates were washed with hot distilled water and dried for 12 h at 90°C before XRD-characterization. The efficacy of metal precipitation was calculated as below:
where, MetalLL and Metalppt are metal contents in leach liquor and precipitates, respectively.
2.4. Solvent Extraction of Cobalt
All the solvent extraction and stripping studies were performed as triplicates. Leach liquor, post Ni precipitation, was used as the aqueous phase stock solution for the Co extraction tests. The stock organic phase was prepared by dilution of a known quantity of Cyanex 272 in kerosene. Each time 50% of the extractant amount into organic phase was saponified by contact with a stoichiometric quantity of NaOH to form the Na salt as per the reaction below [34, 35]:
A 25 mL aliquot of Na-Cyanex 272 stock organic phase was contacted for 5 min, at room temperature (~28 ± 2°C), with the aqueous feed at a unit phase ratio (O/A, 1/1). To determine the extraction isotherm, in some testes the O/A ratios were varied within the range of 1/3 to 3/1. During contact period, the aqueous phase pH was measured and maintained at the equilibrium (as pHeq) by addition of 4.0 M NaOH solution. After the contact time, the phases were separated and the aqueous layer drawn off; metal values in the aqueous phase were determined by ICP. Metal values extracted into the organic phase were calculated by a mass balance between two phases for before and after extraction. Extraction efficacy was calculated by:
where, Metalaq and Metalorg are metals’ concentration in aqueous and organic phases, respectively. Stripping efficacy from the loaded organic phase into a pre-determined volume of 2.0 M H2SO4 solution was calculated as:
3. Results and Discussion
3.1. Leaching Studies
3.1.1. Effect of acid concentration and oxidant addition
The mass transfer of metals from solid cathode powder to an acidic solution is dependent on the lixiviant concentration, where the leach reaction can be described as follows:
The leach behavior of metals from cathode powder was examined by varying H2SO4 concentration over the range of 0.5 M to 3.0 M, whilst the parameters of pulp density (5% w/v), temperature (90°C), and leaching time (180 min) were maintained at constant values. Experimental results presented in Fig. 1(a) suggest a greater degree of leaching with higher acid concentration. Low leach efficiency (62.6% Li, 48.2% Ni, 39.4% Co, and 11.2% Mn) can be improved up to values of ~92% Li and Ni, 68.3% Co, and 34.8% Mn by increasing the H2SO4 concentration from 0.5 M to 3.0 M.
The relatively low leach efficiency of Co and Mn may be due to formation of higher valance state species, as Co3+ and Mn4+, during the charging-discharging cycle of the battery [18]. As per the Eh-pH solution chemistry of both metals, the propensity for dissolution of higher valance states (Co3+ and Mn4+) is reduced relative to the lower valance states (Co2+ and Mn2+). Therefore, in next sets of experiments H2O2 was added as a reductant to reductant to reduce the Co3+ and Mn4+ into their easily leachable forms of Co2+ and Mn2+.
Hydrogen peroxide was added in the range of 0–4 vol.% with 2.0 M H2SO4 lixiviant solution. Although the maximum leach efficiency, without a reductant, was observed with 3.0 M H2SO4 lixiviant solution, the residual free acid concentration post leach was found to be ~1.5 M H2SO4. Therefore, the effect of reductant was investigated with 2.0 M H2SO4 in order to avoid the influence of free acid on leach progress which may mask the reductant effect. The experimental results presented in Fig. 1(b) suggest improved leach efficiency by the aggressive reduction of Co3+ and Mn4+. Leaching of ~99% Co and Mn could be achieved by addition of 4 vol.% H2O2, whereas addition of 0.5 vol.% H2O2 gave leach efficiency of 62% Co and 54% Mn as compared to the leach efficiency of 56.2% Co and 36.4% Mn without reductant.
The leach efficiency of Li (89.2% to > 99%) and Ni (86.6 to > 99%) were also observed to increase with addition of 4 vol.% H2O2. This observation may be interpreted in terms of the close association of some Li and Ni species with higher valance of metal ions, Co3+ and Mn4+, which require a reductive environment for their efficient liberation from the mineral matrix [27]. The leach reaction in the presence of H2O2 can be described by:
3.1.2. Effect of temperature
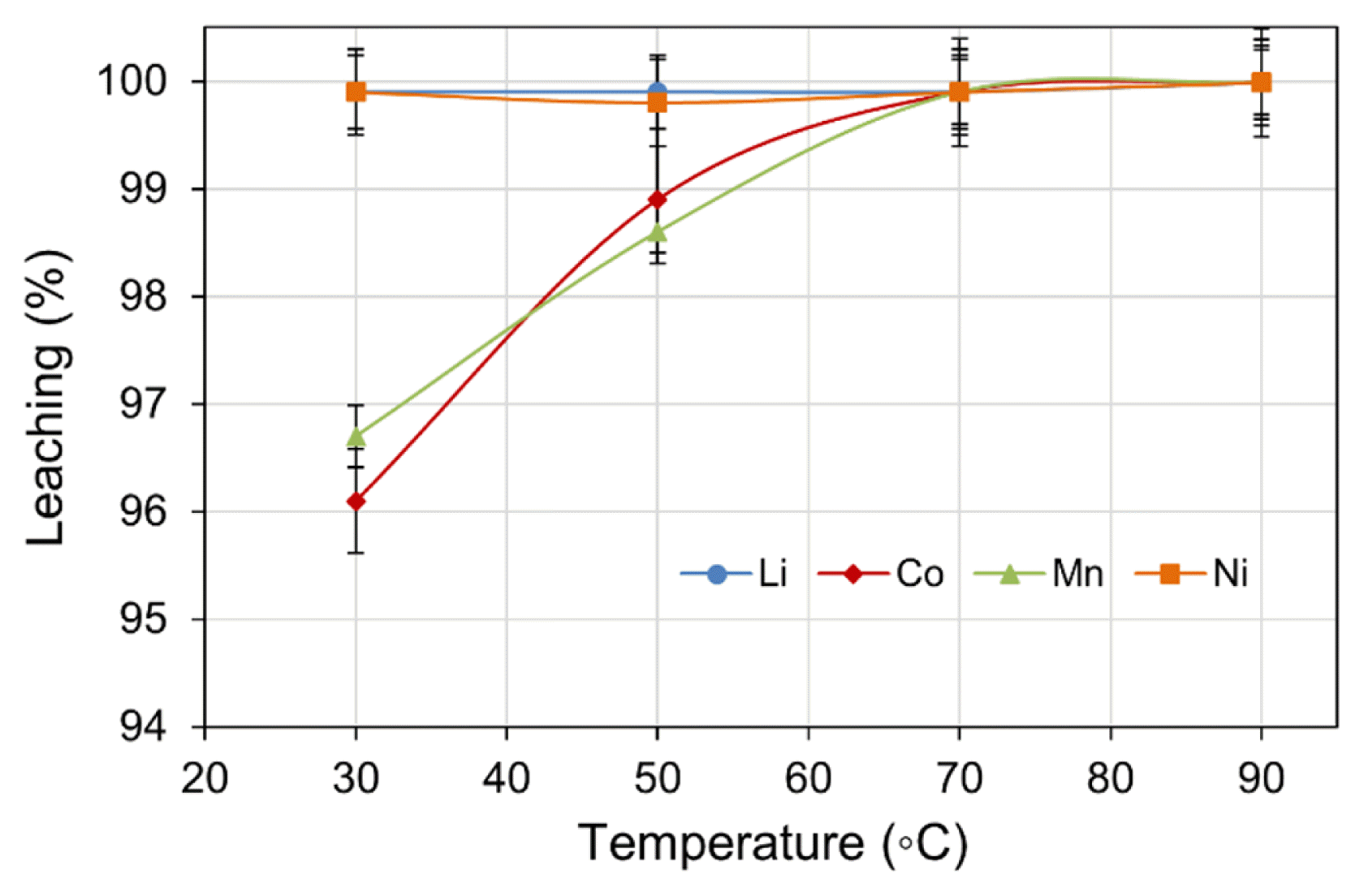
The effect of temperature on metal leach efficiency was investigated within the range of 30–90 (± 2)°C. Other experimental parameters: H2SO4 concentration (2.0 M), H2O2 dosage (4 vol.%), pulp density (5% w/v), and leach time (180 min) were maintained at constant values. The results shown in Fig. 2 suggest that temperature did not significantly affect the leach efficiency of Li and Ni, however, the leach efficiency of both Co and Mn improved to 99.9% when the temperature was held at ≥ 70°C. The effect of temperature can be described by a shift to leach kinetics from diffusion-control to chemically-control region [36]. The change in temperature also affects the thermodynamic boundaries for the reduction of Co3+ and Mn4+ by the enlarged overlapping of MnO2/Mn2+ and Co(OH)3/Co2+ in the predominance region [37]. Although the leach efficiency percentage appears similar, the absolute metal values in the leach liquor were significantly different being 708 mg/L and 483 mg/L for Co and Mn, respectively. When considered in terms of energy input and leach efficiency, 70°C was the optimal leach temperature.
3.1.3. Effect of time
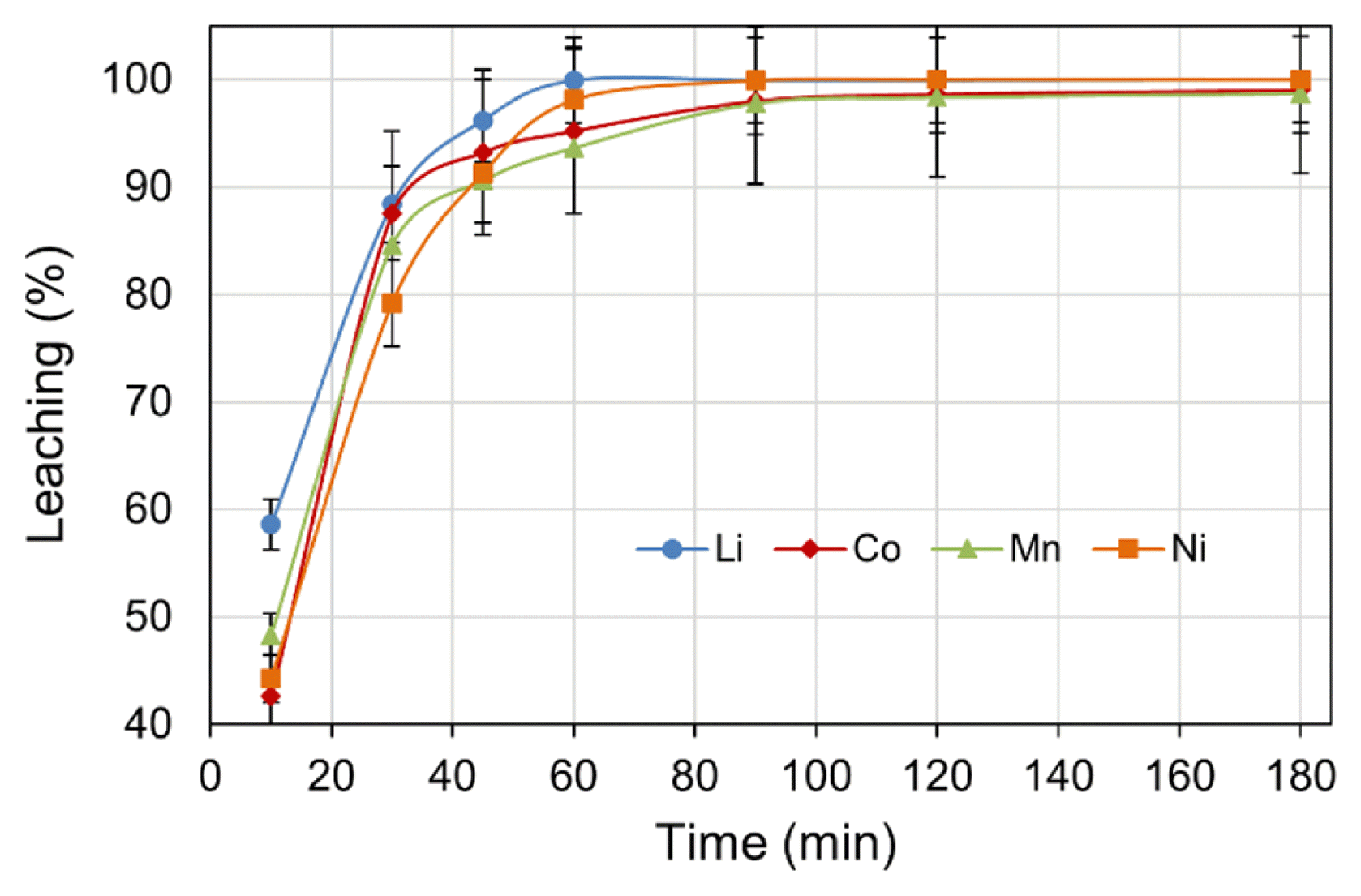
Further, the effect of leach time, up to a maximum of 180 min on metals leaching were examined whilst maintaining the experimental parameters of: H2SO4 concentration (2.0 M), H2O2 dosage (4 vol.%), pulp density (5% w/v), and temperature (70°C) at constant values. The results presented in Fig. 3 indicate that metal leach rapidly increased within the initial 10 min, then slowed to a low rate. The decrease in leach rate may be due to consumption of lixiviant acid by metal dissolution as the leach reactions progress [37]. A variance in the progress of initial leaching, mainly between Li and other metals, was observed. After the initial 10 min of leaching, 58.6% Li was leached; whereas the percentages of 48.3% Mn, 44.3% Ni, and 42.6% Co were somewhat lower. The higher leach rate of Li in the initial stage may be due to the difference in valance state of Li+ relative to the other heavier metals [33]. The relatively low leach efficiencies of Co and Mn at the initial stage is likely attributed to the kinetics of conversion of higher valance metals into their reduced lower state for easy leach in the acidic solution. The overall leach efficiency was found to increase with respect to time, reaching > 90% for all the metals within 45 min of leaching. The optimal leach period was determined to be 150 min, at which > 99% leach efficiency for all metals was achieved.
3.2. Recovery of Rare Metals
3.2.1. Nickel precipitation with DMG
The separation of Ni and Co is usually undertaken by solvent extraction using Cyanex 272. A high value of separation factor (βCo/Ni) can be obtained at low Ni:Co concentration ratio in the pregnant solution [38], otherwise, a similar extraction behavior (pH0.5) can be observed for both metals [35]. In the present study, the similar concentrations of both metals were in the feed solution make it difficult to achieve a large βCo/Ni value, hence, the complexation-precipitation of Ni-DMG was investigated for selective recovery of Ni. The complexation-precipitation reaction for Ni-DMG can be described by:
Leach liquor post Mn precipitation, described in section 2.3 with pH adjusted to 5.0, was used as the stock solution for this study. The ratio of DMG:Ni2+ was varied in the range of 0.5:1 to 3:1, the tests were performed at 80°C. The analysis results obtained from samples taken 60 min from the addition of DMG and presented in Table 1(a). From these results a high selectivity is observed for Ni-DMG complexation over Co2+ and Li+. Approximately 99% of Ni was precipitated at the DMG:Ni2+ ratio of 2:1 which was only ~57% when the stoichiometric amount of DMG was introduced into the solution. The selective precipitation of Ni as Ni-DMG complex is advantageous due to its easy dissolution in acid solutions to facilitate DMG recycle and Ni recovery [34, 39].
3.2.2. Solvent extraction of cobalt with Na-Cyanex 272
Co extraction was undertaken by use of 0.64 M organic solution of Cyanex 272 in which 50% of the extractant molecules was salted as Na-Cyanex 272. The Ni-stripped leach liquor was used as the stock feed for the test work. The extraction reaction can be written as:
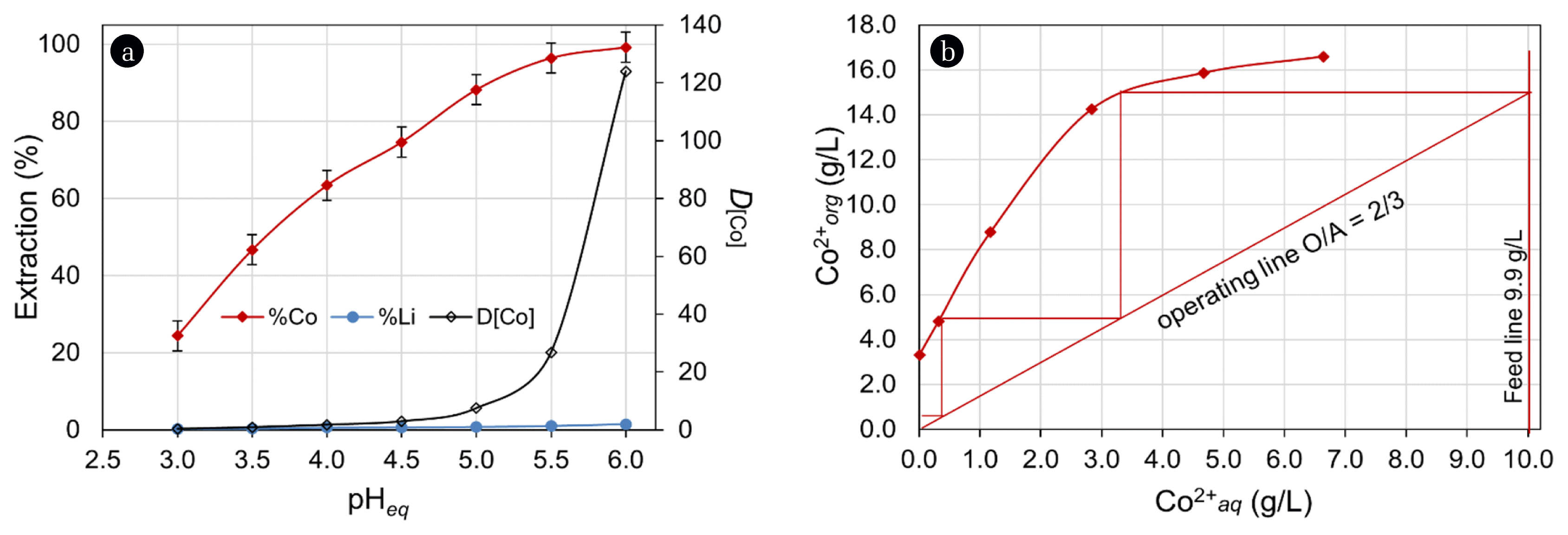
The effect of equilibrium pH (pHeq) was investigated within the range of 3.0–6.0 at an O/A ratio of 1/1. The results are presented in Fig. 4(a) from which it can be observed that extraction of Co improved with increased pHeq. An extraction value of 99% was attained at pHeq 6.0, whilst an extraction of 24.4% was obtained at pHeq ~3.0. Within the varied of pHeq, the distribution of Co (DCo) between the aqueous and organic phases increased from 0.3 at pHeq 3.0 to > 124 pHeq 6.0. However, Li extraction also increased above pHeq 5.0, being > 3% at pHeq 6.0. Such phenomena can be corroborated by the order of stability below olation in the aqueous phase and a greater hydrolysis constant (pKa) of Co2+ than that of Li+ speciation of metal ions [40–43]. At the optimal pHeq 5.0, extraction efficiencies of Co and Li were 88.2% and 0.7%, respectively. These efficiencies were deemed to be inadequate, consequently variation in O/A ratio was investigated for the quantitative extraction of Co. The O/A ratio was varied within the range of 3/1 to 1/3 at pHeq 5.0. It was observed at O/A ratio of 3/1, Co extraction increased up to 99.9% with a single stage contact. Using the results of O/A variation, a McCabe-Thiele diagram (Fig. 4(b)) was constructed to determine the extraction isotherm of Co. It indicated that the experiment carried out at O/A ratio of 2/3 would require 3-contact stages for the quantitative extraction from a feed containing 9.96 g/L Co. Further, the extraction tests carried out at O/A of 2/3 in batch counter-current, confirmed the quantitative extraction of Co, leaving only 8 mg/L Co in the raffinate.
The recovery of Co into an aqueous phase was carried out by stripping the organic phase with a sulfuric acid solution. The stripping reaction can be described by:
In order to get a concentrated CoSO4 solution, the metal loaded organic was contacted with 2.0 M H2SO4 at a higher O/A of 8/1 for 10 min duration. The resultant CoSO4 solution was of 114 g/L Co which was subsequently crystallized to yield crystals of high purity CoSO4.xH2O.
3.2.3. Lithium precipitation with Na2CO3
Prior to precipitation by addition of Na2CO3, the volume of raffinate collected after solvent extraction of Co was reduced by evaporation to double the initial concentration of Li from 3.62 g/L. Literature reports disclosed that solubility of Li2CO3 decreases at elevated temperatures; on this basis, the precipitation reactions were investigated at ~100°C [18, 34]. The effect of precipitant (Na2CO3) dosage was studied within range of 0.5 M to 1.5 M Na2CO3 for 60 min of duration. The results presented in Table 1(b) indicate that an increased ratio of Na2CO3:Li+ could enhance the precipitation yield. Li precipitation was ~94% at the stoichiometric molar ratio of 1:1. Efficiency improved to ~99% at a Na2CO3:Li+ ratio of 1.2:1, which was identified as optimum for Li recovery. A higher dosage of Na2CO3 optimized in previous work [34], suggests for an increasing probability of contact between the carbonate anions and metal cations. The precipitation reaction can be described by:
3.3. Characterization of Recycled Products
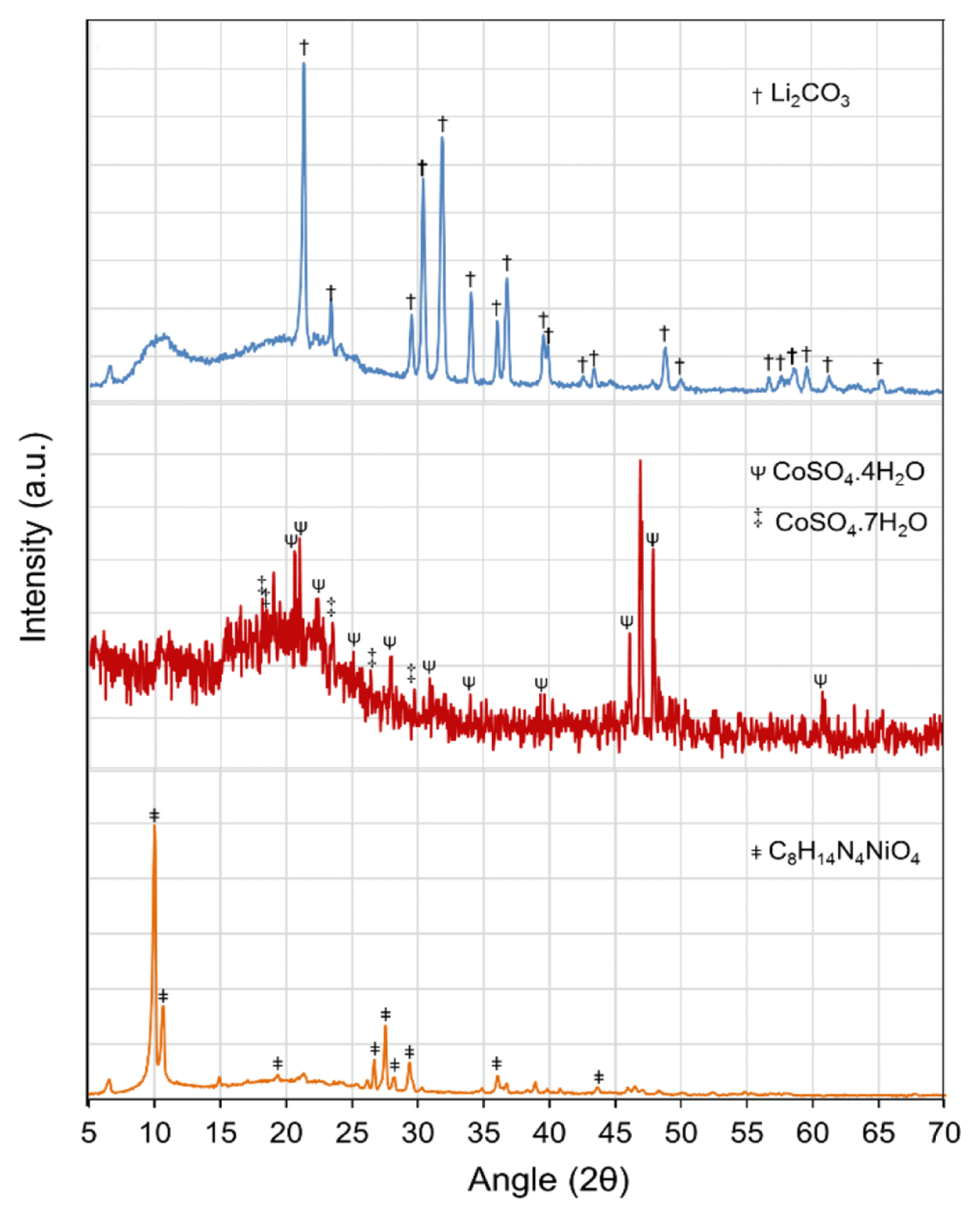
All the recovered precipitates and crystal products were characterized by XRD analysis, and presented in Fig. 5(a)–(c). The XRD patterns revealed the formation of C8H14N4NiO4 (Ni-DMG, Fig. 5(a)), CoSO4.xH2O (Fig. 5(b)), and Li2CO3 (Fig. 5(c)). A wet-chemical analysis of the products was performed which results indicate values of 20.2% Ni, 20.5% Co, and 10.1% Li in the Ni-DMG precipitate, hydrated crystals of CoSO4, Li2CO3 precipitate, respectively.
3.4. Economic Analysis and Flow-sheet Development
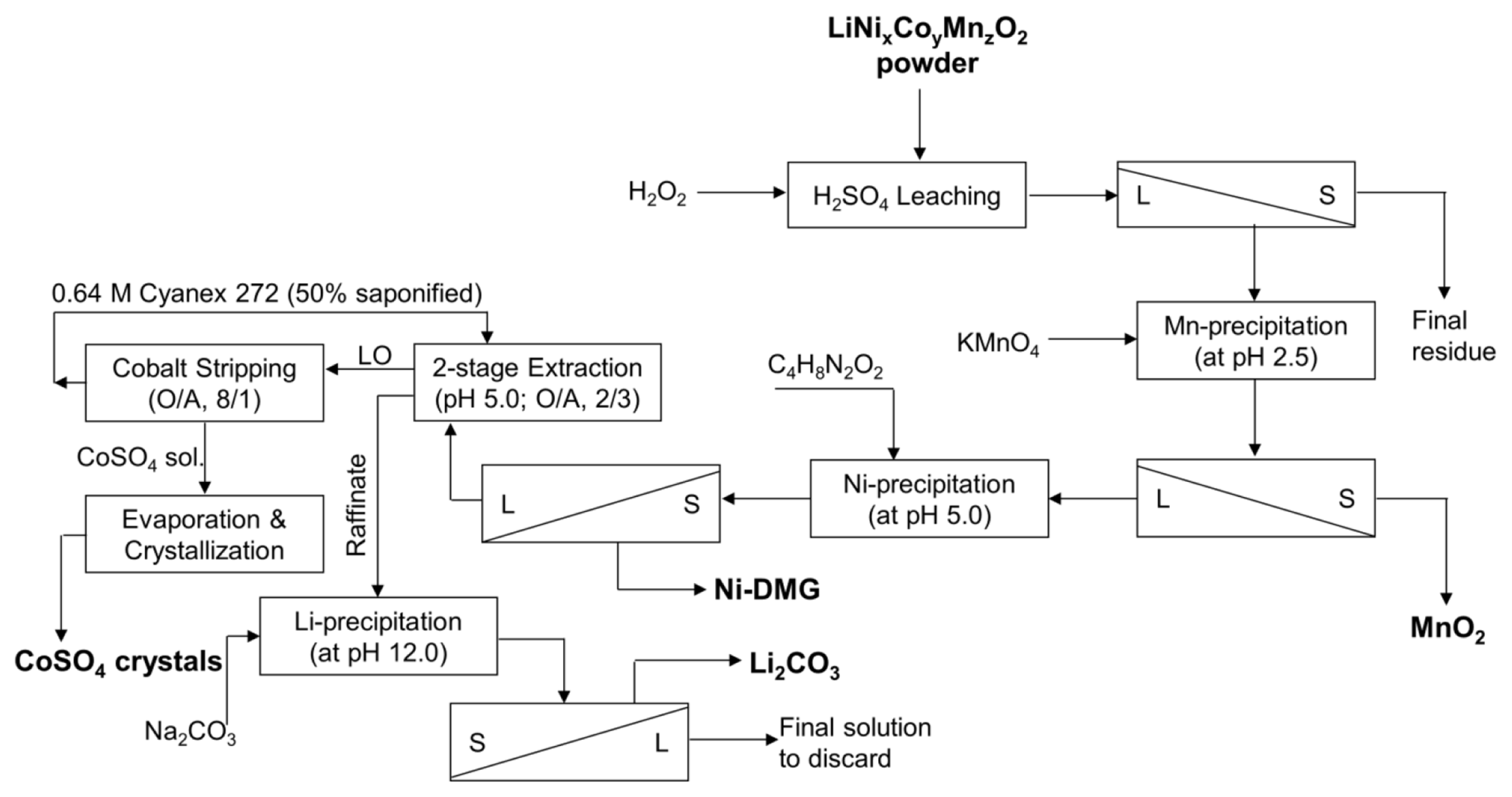
The viability of any industrial process is dependent on process economics, unless there is sufficient margin of profit novel processes developed in the laboratory are seldom adopted by industry. To determine if the metal recovery strategy described above is a viable economic proposition, a brief analysis was conducted using the optimized process parameters for recycling one ton of EoL-LiBs cathode material following the process flow-sheet presented in Fig. 6.
The costs of the hydrometallurgical recycling unit processes that include: leaching, precipitation, solvent extraction steps were calculated. The standard average reagent price used for the analysis is presented in Table 2. The costs of handling, dismantling, labor, and energy usage are considered as reported elsewhere [44]. Table 2 inferred that the process developed by this study is an economically viable process, with an estimated margin of profit $476.1 per ton of cathode powder. It is likely that this margin can be increased for large scale operations due to economy of scale.
4. Conclusions
The potential of rare metals recovery from the EoL-LiBs cathode material has been investigated using the hydrometallurgical techniques of leaching, precipitation, and solvent extraction. The efficiency of a H2SO4 leach was found to increase by addition of the reductant H2O2 to the leach solution. Approximately 99% metal values of interest Ni, Co, and Li could be recovered from the cathode material at the optimized process conditions of 2.0 M H2SO4, 4 vol.% H2O2, 5% pulp density, 70°C, and 150 min leach time. A selective recovery of Ni (~99%) was achieved by addition of DMG to the leach liquor at a 2:1 DMG:Ni2+ molar ratio. Subsequently, a quantitative separation of Co was carried out using a 3-stage solvent extraction with 0.64 M Cyanex 272 (50% as Na-Cyanex salt) at an O/A ratio of 2/3 at pHeq ~5.0. Co stripping from the metal loaded organic phase was successfully performed with 2.0 M H2SO4 solution at a higher O/A of 8/1. The stripped CoSO4 solution, contained 114 g/L Co, was crystallized to recover high purity crystals of CoSO4.xH2O. The final step of process strategy is the precipitation of Li at 100°C with a 1.2:1 molar ratio of Na2CO3 to Li+. In addition, the process economics were briefly examined, the cost was calculated for each unit operation of the hydrometallurgical process, reagent costs, labor and other factors were considered, the overall profit margin per ton of cathode material is estimated to be $476.