AbstractThe current paper reports on a newly developed DBD-Mn/FCRB hybrid system to explore the removal of NOx by reduction without adding reducing gas at low temperature (below 80°C). This technology was established with a fixed carbon-based reduction catalytic reduction bed loaded with manganese (Mn/FCRB) induced by dielectric barrier discharge (DBD). The NO conversion and N2 selectivity in the new hybrid system reached 90.9 and 79.9%, respectively under 8% oxygen content, 1,200 J/L specific input energy (SIE), which were all higher than in the single DBD and DBD-FCRB systems, respectively. The Mn/FCRB was further characterized before and after activation by SEM, XRD and XPS. The possible reaction pathways of denitration were proposed through three processes based on the experimental results: direct denitration of active carbon atoms excited by plasma, reduction by adsorptive C(N) and C(O) complexes on the FCRB surface, and the reaction of nitrogen oxides with by-product CO. In addition, the results also showed that the new in-situ reduction denitration system had strong oxygen shock resistance and water resistance.
1. IntroductionWith the increasing awareness of environmental protection, more and more attention has been paid to the atmospheric environment. Nitrogen oxides (NOx) are considered the primary pollutants worldwide which come from combustion processes of coal-based fossil fuel power generation, fuel vehicles and other incineration processes [1–3]. They are responsible for such environmental problems as photochemical smog [4, 5], acid rain [6], combining with volatile organic compounds to produce ozone [7]. Further to the above, they cause many health problems including damage to the heart and lungs [8–10]. Nowadays, NOx are mainly removed by selective catalytic reduction (SCR) owing to its high removal efficiency [11, 12]. But this technology features a relatively narrow operation temperature window (300–400°C) which means that the de-NOx unit is placed upstream of the dedusting and desulfuration units, leading to activity declining and shorter service life of the catalysts [13]. What’s more, the SCR technology continues to raise the worries for the leakage or escape of the reducing gas (for example, ammonia slip) [14]. Therefore, an environment-friendly de-NOx technology at low temperature has long been a question of great interest.
The non-thermal plasma (NTP) has been reported as a promising and effective technology for removing NOx which generally consists of highly reactive species including electrons, ions, radicals, excited and neutral species [15, 16] that all can interact with each other and contribute to the decompose hazardous air pollutants. NTP is used to selectively transfer energy to the electrons, thus avoiding the energy consumption necessary for the heating of the entire gas flow and the strong temperature dependence of the catalytic activity can be overcome by the addition of the plasma technology [17, 18]. Dielectric barrier discharge (DBD) has been demonstrated as the superior mode for the generation of NTP [19, 20]. Nevertheless, NO removal by plasma was mainly attributed to oxidation [21–25], hence ammonia [26, 27] and hydrocarbon gas as CH4 [28, 29], C3H6 [30], C3H8 [31], C7H16 [32] and C8H18 [33] are used as reducing gases in many previous studies. However, there is the limitations of gas leakage are yet to be overcome.
Carbonaceous materials can reduce NOx as an adsorbent and undergo heterogeneous gas-solid reactions with NO to form N2 [34]. Moreover, plasma-enhanced catalysis can lead to synergistic effects [35, 36] and induce the C-NO reaction which requires a very high temperature (above 900°C) in general [37, 38]. However, due to the high affinity of carbon to oxygen [39, 40], carbon will preferentially react with residual O2, resulting in low N2 selectivity and high carbon combustion [41]. Manganese, as one of the transition metals, is often used as the active component of catalysts because of its abundant variable state including MnO, MnO2, Mn2O3, Mn3O4, Mn5O8 [42–44], and many studies have proved the important contribution of Mn as catalysts for NOx reduction by carbon materials [42, 43, 45–47]. Therefore, the method used fixed carbon-based reduction beds (FCRB) loaded with Mn induced by DBD is expected to become a new denitrification technology at low temperature which has low carbon consumption and high N2 selectivity. However, there is no research on this technology, and the specific reaction pathways for the system have not been figured out owing to the rapid, complex and undetectable reaction process.
The current paper reports on a new developed system utilized to investigate the performance of the DBD induced Mn/FCRB (DBD-Mn/FCRB) de-NOx technology at low temperature (below 80°C), compared with the single DBD system and the DBD-FCRB hybrid system. And the pore structure and surface micro-characteristic parameters of the Mn/FCRB before and after activation were also measured to explore the specific reaction mechanism of denitration. In addition, the influence of oxygen content and humidity on the denitration effect of the new system has been further investigated.
2. Material and Methods2.1. Experimental SetupIn this paper, industrial coal-based activated carbon was purchased from Heatton Environmental Tech Ltd. (Shanghai, China), and the performance parameters of the FCRB are shown in Table S1. After grinding and mechanically sieving the original sample, the activated carbon with a particle size distribution of 0.6–1 mm (18–30 mesh) was obtained, which was washed 3 times with deionized water to remove the original impurities and dried at 105°C for 12 h.
The Mn/FCRB catalyst was introduced by excess-solution impregnation using manganese acetate ((CH3COO)2Mn) of appropriate concentration to obtain around 5 wt% metal content (10 ml of solution/gram of FCRB), and the mixed solution was placed on a magnetic stirrer for 3 h. The FCRB-solution mixtures were dried and placed in an oven for 10 h at 110°C, then the dried product was put into a muffle furnace and calcined at 450°C for 3 h.
The denitration reaction device is shown in Fig. 1, which mainly includes the gas supply system, reaction system, and outlet gas analysis and detection system. The experiment was carried out in a dielectric barrier discharge reactor with a coaxial cylinder structure, including an inner tube (quartz glass, 11 mm outer diameter, tube length 275 mm, wall thickness 1 mm), outer tube (stainless steel, inner diameter 21 mm), inner electrode (with a stainless steel sheet wrapped around the inner wall of the inner tube, thickness 0.3 mm, length 165 mm) and the circulating water outer tube (stainless steel, inner diameter 5 mm, tube length 172 mm). The DBD was generated using an alternating current power supply and the specific input energy (SIE) (Eq. (1)) ranges from 600 to 1,200 J/L. A 200 MHz digital phosphor oscilloscope (TDS2024B, Tektronix, USA) was used to analyze power. A probe thermometer (Testo 925, Testo SE & Co. KGaA, Germany) was used to measure the temperature of the FCRB right after each reaction power off, and an infrared thermometer (Testo 830-T1, Testo SE & Co. KGaA, Germany) was used to measure the temperature outside the wall of the reaction zone during each reaction process. The inner tube was hollow for sucking cold air, and the external circulating water ensured that the temperature in the reaction zone was below 80°C. The humidity was adjusted by an ultrasonic atomizer (402AI, yuwell group, China) to control the H2O content in the carrier gas (Argon), therefore the feed gas had different humidity. The humidity was measured by a psychrometer (Testo 605-H1, Testo SE & Co. KGaA, Germany) before the feed gas entered the DBD reactor.
2.2. Experimental Methods300 mg of FCRB or Mn/FCRB was loaded on the quartz wool in the DBD discharge zone, purged with Argon (Ar) at room temperature for 10 min to drive out the remaining impurities, and then the test gases were let into the reactor. To avoid the influence of NOx produced by oxidation of N2 on composition analysis of outlet gas, Ar was selected as the carrier gas which was conducive to the discussion of NOx removal mechanism and reaction paths in the system. NO, Ar and O2 came from the gas tanks containing high purity gases. The inlet flow rate was independently controlled by the Horiba Stec-4400 mass flow controller to ensure that the concentration of NO was 428 mg/m3, and the O2 concentration was controlled at 0–16%. The feed gas flow rate was 2 L/min, the space velocity (Eq. (2)) in the DBD reaction zone was 2,895.2 h−1, and the residence time in the reactor was 2.1 s. After the NOx monitor (Testo 340, Testo SE & Co. KGaA, Germany) detected that the NOx concentration in the outlet gas was stable, the plasma power supply was switched on to start the discharge and the FTIR spectrometer (FTIR 850, Guangdong Co., China, 0.5 cm−1) was used to quantitatively analyze the NOx components of the outlet gas, and the data were all collected 15 min after the reaction began when the outlet NOx concentration had stabilized. The gas chromatography (GC-9860, Jump Instrument, China) was used to detect the CO and CO2 content in the outlet gas.
The space velocity, removal performance and N2 selectivity of the DBD-Mn/FCRB hybrid system were defined as follows:
Where NOin, NOout, NO2 in and NO2 out are the inlet and outlet concentrations of NO and NO2, respectively, ppm. NO2 in all came from the NO oxidation in presence of O2 in the intake pipe.
2.3. CharacterizationA scanning electron microscope (SEM, SU8010, Hitachi, Japan) was carried out for the surface morphology change of Mn/FCRB before and after DBD activation. X-ray powder diffraction (XRD) measurements were performed using Automated Multipurpose X-ray Diffractometer (Ultima IV, Rigaku, Japan). The XRD diffraction patterns were taken from 10° to 80° of 2θ at a scan rate of 10°/min. X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Scientific, USA) experiments were carried out with Al Kα radiation (1486.6 eV). Binding energies were calibrated using the C1s peak (284.6 eV) as a reference.
3. Results and Discussion3.1. De-NOx PerformancesAs shown in Fig. 2, when the plasma SIE increased from 600 to 1,200 J/L, the NO conversion and N2 selectivity improved with the enhancement of the discharge energy in the single DBD, DBD-FCRB and DBD-Mn/FCRB systems. The NO conversion and N2 selectivity in the single DBD system were only 52.9 and 33.0%, respectively under 8% oxygen, 1,200 J/L SIE, while those in the DBD-FCRB system reached 77.7 and 66.4%, respectively, and the highest NO conversion and N2 selectivity were increased to 90.9 and 79.9% in the DBD-Mn/FCRB system, respectively. Therefore, Mn loading can serve reduction denitrification as the dominant denitration path, and significantly improve its denitrification performance and N2 selectivity. Moreover, compared with the NH3-SCR system using carbon-based loaded Mn catalyst without NTP activation at low temperature, the NO conversion of these technologies is usually less than 80%, and the temperature is about 160–250°C [48, 49]. And the NO conversion is generally in the range of 50–75% when the temperature is as high as 150–350°C in the NTP-stimulated SCR denitrification technology with different catalysts at low temperature [50, 51].
In the single DBD system, plasma collided with NO, O2, and other molecules through electrons, thereby generating free radicals such as N and O· that can oxidize or reduce NO (Eq. (5)~(8)). In the DBD-FCRB and DBD-Mn/FCRB systems, the reactions described above also occurred, in addition, the carbon activated by DBD may react with NO (Eq. (9) and (10)). As a result, the excellent N2 selectivity was performed through the reduction denitration path.
3.2. Mn/FCRB Characterization3.2.1 SEM results
Fig. 3 shows the SEM images of the original blank FCRB (B-FCRB), the FCRB and Mn/FCRB activated by DBD (A-FCRB and A-Mn/FCRB). After the plasma activation, there were obvious traces of etching both on the surfaces of A-FCRB and A-Mn/FCRB. These findings suggest that the surface of the FCRB and Mn/FCRB was excited by DBD to produce short-lived active carbon atoms. The previous researches have shown that through the high-energy gas species penetrating the surface of the material to a depth of several nanometers, the plasma can cause the surface of the carbon material to break the covalent bond, ion impact, etc. so that the surface carbon atoms are removed by various reactions [52, 53]. Thereby, the active carbon atoms excited by plasma etching can probably reduce NO and cause the Eq. (9) and Eq. (10) reactions.
3.2.2. XRD resultsAs shown in Fig. S1, the XRD pattern shows peaks that are identified as SiO2. There was no significant characteristic diffraction peak containing Mn in the sample B-Mn/FCRB, indicating that the active component mainly existed in an amorphous state, and SiO2 is decomposed by calcination in the preparation process. Higher dispersion can inhibit the formation of crystal clusters, effectively reduce the particle size of manganese oxide on the catalyst surface, improved the dispersion degree of active component MnOx on the FCRB and increased lattice defects of the catalyst [54]. The surface distribution uniformity of Mn/FCRB decreased after DBD activation, and there are obvious characteristic peaks of MnOx in the sample A-Mn/FCRB.
3.2.3. XPS results
Fig. 4(a) shows the surface elements of B-FCRB, A-FCRB, B-Mn/FCRB and A-Mn/FCRB were mainly carbon, nitrogen, and oxygen. The relative contents of the elements and functional groups are listed in Table 1. As can be seen from the table, the C1s/O1s atomic ratio has decreased from 8.4 (B-FCRB) and 1.7 (B-Mn/FCRB) to 7.5 (A-FCRB) and 1.6 (A-Mn/FCRB) respectively, indicating that oxygen functional groups (OFGs) have increased after the plasma activation. The C1s/N1s atomic ratio has increased from 44.7 (B-FCRB) and 47.7 (B-Mn/FCRB) to 51.6 (A-FCRB) and 51.9 (A-Mn/FCRB) respectively after plasma activation, showing that nitrogen functional groups (NFGs) were consumed during the denitration process.
The C1s peaks of B-FCRB and A-FCRB were divided into 5 peaks respectively, as shown in Fig. 4(b). As can be seen from Table 1, the intensity of C=C groups reduced after DBD activation from 54.5 to 51.2%. It is known that the energy of positive, negative ions and electrons present in the plasma is sufficient to break chemical bonds on the outer surface of a material, and the radicals formed in the plasma process were first formed in the π bonds (C=C) on the surface of carbonaceous materials because they are active and the most susceptible to plasma attack [55–57]. Therefore, the carbon-carbon network was disrupted, and new C(O) and C(N) groups were generated on the surfaces of Mn/FCRB by DBD activation as shown by Eq. (11)~(13) (where C* means the active site on the Mn-FCRB surface). The results show that the total content of C(O) complexes including C-O (e.g., epoxy, hydroxyl), C=O (e.g., carbonyl, quinone), and C(O)O (e.g., carboxyl, lactone) was increased from 42.9 to 46.8%. However, the content of C-O and C-N was decreased after the denitration process. This result may be explained by the fact that these two complexes are more unstable and more likely to participate in the reactions and be consumed.
The importance of C(N) and C(O) complexes in the C-NO reaction system was often emphasized [58–61], and the same considerations may be indicated in the C-NO induced by DBD. C(N) can react with newly adsorbed NO near the surface of Mn-FCRB to produce N2 in a gas-solid heterogeneous phase, as shown by Eq. (14). Moreover, C(N) can also produce N2 through the solid-phase polymerization reaction of Eq. (15), which also has the effect of reducing denitrification and will re-release carbon active sites. On the other hand, C(O) complexes were beneficial to the electron transfer on the carbon surface. Moreover, C(O) has high reactivity that can directly reduce NO and the reduction reaction between C(O) and NO is shown in Eq. (16). As NO is reduced to N2, the active sites of carbon resume activity again, and it goes back and forth.
The Mn2p of Mn/FCRB were divided into three peaks: Mn2+, Mn3+ and Mn4+, and the peaks were near 640.1, 641.4 and 642.5 eV, respectively [62–64]. As shown in the Table 1, the proportion of third- and fourth-valence Mn increased after the DBD activation, indicating that DBD treatment was beneficial to increase the proportion of the high-valence component of the catalyst. The oxygen vacancies are known to play an important role in the adsorption and dissociation of oxygen molecules, leading to the generation of highly active electrophilic O2 [65]. In O1s XPS spectra (Fig. 4(d)) of B-Mn/FCRB and A-Mn/FCRB, the peak at 531 eV is assigned to lattice oxygen (Oβ), and the peak in B-Mn/FCRB and A-Mn/FCRB occur at 532 eV, which can be assigned to surface adsorbed oxygen species (Oα), resulting from the adsorption of gaseous O2 into oxygen vacancies [66]. The Oα/Oβ ratio increased after DBD activation (Table 1), in good agreement with the de-NOx activities, which also indicated that DBD activation increased oxygen vacancies that promoted superior low-temperature reducibility.
3.3. COx ProductionAs shown in Fig. 5(a), after the injection of NO, CO outlet concentration has increased from 2.2 to 15.4 mg/m3, and the CO2 outlet concentration has also increased from 52 to 126 mg/m3 in the DBD-FCRB system in the absence of oxygen. This can lead to the conclusion that the amount of COx produced has increased significantly due to the input of NO, which not only proved that C will react with NO (because O of NO was the only source of O in COx in the absence of oxygen), also proved the existence of CO products in the reaction zone just as the presumed Eq. (10). An implication of this result is the possibility that there should be a gas-phase reduction reaction Eq. (17) in the DBD-FCRB system.
To further prove the role of the CO-NO reaction, the CO concentration measurement under the 8% oxygen condition was carried out. As shown in Fig. 5(b), (c), the amount of CO and CO2 produced increased with the SIE increased, but the CO output in the presence of NO was less than in the absence of NO and CO2 output presented an opposite trend. If NO was only reduced and removed by C(O) and C(N) complexes or active carbon atoms, then due to the reactions of Eq. (9), (10), and (14) happening, theoretically the CO content should increase in the presence of NO, which was contrary to the experimental results. The results indicated that CO was consumed by the NO-CO reaction in the DBD-Mn/FCRB system. What’s more, when NO was present in the gas intake, the effect of Mn catalyst was to significantly increase the reactivity both by increasing the number of reaction sites via the catalyst dispersion and reducing the activation energy and by increasing CO2 production [67]. The latter can also be proved by the average CO2/CO ratio that increased from 16.9 to 17.7 compared between the DBD-FCRB system and DBD-Mn/FCRB system, indicating that less carbon was consumed per molecule of NO reduced in the DBD-Mn/FCRB system.
3.4. Effects of O2 and H2OIt is very necessary to explore the role of oxygen to understand the mechanism of this reduction system. It can be seen from Fig. 6(a) that when the SIE was 800 J/L and the oxygen content increased from 0 to 16%, the NO conversion decreased in the three systems. The large input of oxygen molecules will cause the excitation and decomposition of NO to be weakened and promote the occurrence of oxidation reactions (Eq. (18)~(20)), which increased the concentration of NO and NO2. However, the overall performance of the DBD-Mn/FCRB system was better than that of the single DBD system, especially in the low and medium oxygen zone (4~8%). For the DBD-FCRB and DBD-Mn/FCRB systems, although the reaction atmosphere was undoubtedly an oxidizing atmosphere, there was a reducing system on the surface of FCRB. FCRB can absorb O2 and O· species (Eq. (13)) so that the carbon can act as a buffer for oxygen shock. Therefore, the DBD-FCRB and DBD-Mn/FCRB systems reduced the sensitivity of the system to oxygen concentration. With the further increase of oxygen content, the adsorption active sites on the surface of FCRB gradually approached saturation, its buffering effect on oxygen-containing species was relatively insignificant, but it still had a relatively good denitration effect in the DBD-Mn/FCRB system.
Exploring the influence of humidity on denitration capacity is helpful to the practical application of the technology. As shown in Fig. 6(b), no matter in what relative humidity conditions, the NO conversion of the DBD-FCRB system was better than that of the single DBD system, and DBD-Mn/FCRB was slightly better than the DBD-FCRB system. In the single DBD system, the NO conversion increased from 52.9 to 63.4% then decreased to 42.3%. The increase was caused by H·, ·OH and HO2· active groups generated from water molecules under the bombardment of plasma. As a strong oxidizing radical, HO2· will further oxidize NO to NO2 (Eq. (21)~(23)). And the phenomenon was also found in this study that NO decreased and NO2 increased which also further confirmed this reaction mechanism. The NO conversion in the single DBD system decreased because water molecules acted as a “quencher” gradually, which consumes some of the active species to produce H−, OH− and a few O− anions by dissociative electron attachment to H2O molecules [68], so the number of active species participating in the NO reduction reaction decreased. However, the denitration capacity was stable at high humidity in the DBD/FCRB system and the NO conversion was 87.1% when the relative humidity was 90%, as a consequence of the increased moisture absorption of FCRB as humidity increases [69], and the content of water molecules in the reaction zone was relatively stable. However, although the presence of water vapor did not affect the crystal form, particle size and specific surface area of the Mn-based catalysts, it can compete with NO to adsorb at the active center on the catalyst surface, resulting in a decrease in the number of available active centers in the reaction process, thus inhibiting the activity of the catalyst [70]. Therefore, the NO conversion of the DBD-Mn/FCRB system decreased slightly with the increase of humidity overall and was similar to the DBD-FCRB system in the high humidity area with the lowest 88.0% of the NO conversion.
4. DBD-Mn/FCRB System Reaction MechanismIn the denitration process of the DBD-Mn/FCRB system, the first step is that NO and O2 molecules are impacted by high-energy particles in the plasma atmosphere to produce a large number of active radical groups such as N and O·, and the surface of Mn/FCRB is also etched and excited by the plasma at the same time. Afterward, these gaseous high-energy intermediate particles will undergo adsorption and various chemical reactions with the surface of FCRB.
In summary, the main reduction denitrification reaction processes of the DBD-Mn/FCRB system are shown in Fig. 7. As mentioned earlier, the DBD-Mn/FCRB system is mainly reductive denitrification. The first reductive denitrification path is the direct participation of gas-solid heterogeneous reaction, that is, the Mn/FCRB surface is excited by the strong bombardment of plasma to produce active carbon atoms which can directly reduce NO and produce CO or CO2, as shown in Fig. 7(a). In addition, plasma can also excite more carbon active sites C*, thereby promoting chemical adsorption. The second reaction mechanism is to use the adsorbed C(O) and C(N) complexes to generate solid-phase and gas-solid heterogeneous reactions to reduction denitrification, as shown in Fig. 7(b)~(d). The third path is to use the CO produced by the first two reaction pathways and the desorption of the oxygen complex C(O) on the Mn/FCRB surface for CO-NO gas-phase reaction, as shown in Fig. 7(e).
5. ConclusionsA new hybrid system DBD-Mn/FCRB was established to explore the removal of NOx and the reaction mechanism by reduction without adding reducing gas at low temperature (below 80°C) for the first time. The results presented herein provide definite evidence of the role of Mn/FCRB in promoting the reduction de-NOx performance of the NTP at low temperature. The higher NO conversion and N2 selectivity, and lower carbon consumption in the DBD-Mn/FCRB hybrid system showed the best NO reduction capacity. The mechanisms of the removal of NOx in the new system were also proposed through three processes: direct denitration of active carbon atoms excited by plasma, reduction by adsorptive C(N) and C(O) complexes on the carbon surface, and the reaction of nitrogen oxides with by-product CO. In addition, the new in-situ catalytic reduction denitration system had good oxygen shock resistance and water resistance.
AcknowledgmentsThis research was funded by the technology innovation and level promotion project supported by Shanghai State-owned assets supervision and Administration Commission (No. 2018001) and the science and technology innovation action project supported by the science and technology commission of Shanghai Municipality (No. 18DZ1202605) and the Special research project on causes and control technology of air pollution (No. 2017YFC0212905).
NotesAuthor Contributions F.G. (M.A. student) conducted all the experiments and wrote the manuscript. X.P.J. (M.A. student) assisted in the development of methodology. G.C.W. (Professor) revised the manuscript. L.Y.S. (M.A. student) assisted in conducting experiments. Y.J.T. (M.A. student) assisted in conducting experiments. R.X.Z. (Professor) assisted in formulation of overarching research goals and revised the manuscript. W.X.Z. (Ph.D.) assisted in the manuscript writing. J.Y.H. (Professor) assisted in the development of methodology and data curation. R.N.Z. (Ph.D.) revised the manuscript. References1. Skalska K, Miller JS, Ledakowicz S. Trends in NOx abatement: A review. Sci Total Environ. 2010;408:3976–3989.
4. Bang HQ, Nguyen HD, Vu K, et al. Photochemical smog modelling using the air pollution chemical transport model (TAPM-CTM) in Ho Chi Minh city, Vietnam. Environ Model Assess. 2019;24:295–310.
6. Wang C, Wang W, Sardans J, et al. Effect of simulated acid rain on CO2, CH4 and N2O fluxes and rice productivity in a subtropical Chinese paddy field. Environ Pollut. 2018;243:1196–1205.
7. McDonald BC, De Gouw JA, Gilman JB, et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science. 2018;359:760.
8. Lelieveld J, Evans JS, Fnais M, et al. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature. 2015;525:367–371.
9. Zhao CN, Xu Z, Wu GC, et al. Emerging role of air pollution in autoimmune diseases. Autoimmun Rev. 2019;18:607–614.
10. Schraufnagel DE, Balmes JR, Cowl CT, et al. Air pollution and noncommunicable diseases: A review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air pollution and organ systems. Chest. 2019;155:417–426.
11. Brüggemann TC, Keil FJ. Theoretical investigation of the mechanism of the selective catalytic reduction of nitric oxide with ammonia on H-form zeolites. J Phys Chem C. 2008;112:17378–17387.
12. Tan LA, Guo YA, Liu ZB, et al. An investigation on the catalytic characteristic of NOx reduction in SCR systems. J Taiwan Inst Chem E. 2019;99:53–59.
13. Liang Q, Li J, He H, et al. Effects of SO2 on the low temperature selective catalytic reduction of NO by NH3 over CeO2-V2O5-WO3/TiO2 catalysts. Front Environ Sci Eng. 2017;11:150–156.
14. Dong L, Fan Y, Ling W, et al. Effect of Ce/Y addition on low-temperature SCR activity and SO2 and H2O resistance of MnOx/ZrO2/MWCNTs catalysts. Catalysts. 2017;7:181.
15. Oda T, Kato T, Takahashi T, et al. Nitric oxide decomposition in air by using non-thermal plasma processing-with additives and catalyst. J Electrostat. 1997;42:151–157.
16. Feng X, Liu H, He C, et al. Synergistic effects and mechanism of a non-thermal plasma catalysis system in volatile organic compound removal: a review. Catal Sci Technol. 2018;8:936–954.
17. Palma V, Cortese M, Renda S, et al. A Review about the Recent Advances in Selected NonThermal Plasma Assisted Solid–Gas Phase Chemical Processes. Nanomaterials. 2020;10:1596.
18. Von Keudell A, Volker SVDG. Foundations of low-temperature plasma physics - an introductionJ]. Plasma Sources Sci Technol. 2017;26:113001.
19. Bai M, Zhang Z, Bai M. Simultaneous desulfurization and denitrification of flue gas by ·OH radicals produced from O2
+ and water vapor in a duct. Environ Sci Technol. 2012;46:10161–10168.
20. Kogelschatz U. Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma P. 2003;23:1–46.
21. Zhu AM, Sun Q, Niu JH, et al. Conversion of NO in NO/N2, NO/O2/N2, NO/C2H4/N2 and NO/C2H4/O2/N2 systems by dielectric barrier discharge plasmas. Plasma Chem Plasma P. 2005;25:371–386.
22. McLarnon CR, Penetrante BM. Effect of gas composition on the NOx conversion chemistry in a plasma. SAE transactions. 1998;107:886–897.
23. Stefanovic I, Bibinov NK, Deryugin AA, et al. Kinetics of ozone and nitric oxides in dielectric barrier discharges in O2/NOx and N2/O2/NOx mixtures. Plasma Sour Sci Technol. 2001;10:406–416.
24. Yan K, Kanazawa S, Ohkubo T, et al. Oxidation and reduction processes during NOx removal with corona-induced nonthermal plasma. Plasma Chem Plasma P. 1999;19:421–443.
25. Malik MA, Kolb JF, Sun Y, et al. Comparative study of NO removal in surface-plasma and volume-plasma reactors based on pulsed corona discharges. J Hazard Mater. 2011;197:220–228.
26. Zhu T, Zhang X, Niu W, et al. Selective catalytic reduction of NO by NH3 using a combination of non-thermal plasma and Mn-Cu/ZSM5 catalyst. Catalysts. 2020;10:1044.
27. Jiang B, Zhao S, Wang Y, et al. Plasma-enhanced low temperature NH3-SCR of NOx over a Cu-Mn/SAPO-34 catalyst under oxygen-rich conditions. Appl Catal B. 2021;286:119886.
28. Zhu T, Zhang X, Yi N, et al. NOx storage and reduction assisted by non-thermal plasma over Co/Pt/Ba/γ-Al2O3 catalyst using CH4 as reductant. Plasma Sci Technol. 2021;23:025506.
29. Pan H, Guo Y, Jian Y, et al. Synergistic effect of non-thermal plasma on NOx reduction by CH4 over an In/H-BEA catalyst at low temperatures. Energ Fuel. 2015;29:5282–5289.
30. Wang X, Li Y, Chen W, et al. Characteristics of NOx removal combining dielectric barrier discharge plasma with selective catalytic reduction by C3H6
. Jpn J Appl Phys. 2010;49:086201.
31. Pan H, Qiang Y. Promotion of non-thermal plasma on catalytic reduction of NOx by C3H8 Over Co/BEA catalyst at low temperature. Plasma Chem Plasma Process. 2014;34:811–824.
32. Nguyen DB, Nguyen VT, Heo IJ, et al. Removal of NOx by selective catalytic reduction coupled with plasma under temperature fluctuation condition. J Ind Eng Chem. 2019;72:400–407.
33. Stere CE, Adress W, Burch R, et al. Probing a non-thermal plasma activated heterogeneously catalyzed reaction using in situ DRIFTS-MS. Acs Catal. 2015;5:956–964.
34. Gupta H, Benson SA, Fan L, et al. Pilot-Scale Studies of NOx Reduction by Activated High-Sodium Lignite Chars: A Demonstration of the CARBONOX Process. Ind Eng Chem Res. 2004;43:5820–5827.
35. Ollegott K, Wirth P, Oberste-Beulmann Christian, et al. Fundamental Properties and Applications of Dielectric Barrier Discharges in Plasma-Catalytic Processes at Atmospheric Pressure. Chemie Ingenieur Technik. 2020;92:1542–1558.
36. Blackbeard T, Demidyuk V, Hill SL, et al. The Effect of Temperature on the Plasma-Catalytic Destruction of Propane and Propene: A Comparison with Thermal Catalysis. Plasma Chem Plasma Process. 2009;29:411.
37. Zhao W, Lian L, Jin X, et al. In situ electron-induced reduction of NOx via CNTs activated by DBD at low temperature. Front Environ Sci Eng. 2020;14:20.
38. Peng M, Zhao R, Xia M, et al. Study on the mechanism of NO removal by plasma-adsorption catalytic process. Fuel. 2017;200:290–298.
39. Illán-Gómez MJ, Raymundo-Piñero E, García-García A, et al. Catalytic NOx reduction by carbon supporting metals. Appl Catal B. 1999;20:267–275.
40. Illán-Gómez MJ, Salinas-Martínez de Lecea C, Linares-Solano A, et al. Potassium-containing coal chars as catalysts for NOx reduction in the presence of oxygen. Energ Fuel. 1998;12:1256–1264.
41. Illán-Gómez MJ, Brandán S, Linares-Solano A, et al. NOx reduction by carbon supporting potassium-bimetallic catalysts. Appl Catal B. 2000;25:11–18.
42. Liu C, Shi J, Gao C, et al. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl Catal A-Gen. 2016;522:54–69.
43. Raja S, Alphin MS, Sivachandiran L. Promotional effects of modified TiO2- and carbon-supported V2O5- and MnOx-based catalysts for the selective catalytic reduction of NOx: a review. Catal Sci Technol. 2020;10:7795–7813.
44. Han L, Cai S, Gao M, et al. Selective catalytic reduction of NOx with NH3 by using novel catalysts: state of the art and future prospects. Chem Rev. 2019;119:10916–10976.
45. Illán-Gómez MJ, Linares-Solano A, Radovic LR, et al. NO reduction by activated carbons. 7. Some mechanistic aspects of uncatalyzed and catalyzed reaction. Energ Fuel. 1996;10:158–168.
46. Li P, Lu P, Zhai Y, et al. Low temperature SCR of NO with catalysts prepared by modified ACF loading Mn and Ce: effects of modification method. Environ Technol. 2015;36:2390–2400.
47. Xu G, Guo X, Cheng X, et al. A review of Mn-based catalysts for low-temperature NH3-SCR: NOx removal and H2O/SO2 resistance. Nanoscale. 2021;13:7052–7080.
48. Yanli W, ChuanZhang G, Liang Z, et al. MnOx–CeO2/Activated Carbon Honeycomb Catalyst for Selective Catalytic Reduction of NO with NH3 at Low Temperatures. Ind Eng Chem Res. 2012;51:11667–11673.
49. Lishan W, Bichun H, Yanxia S, et al. Manganese oxides supported on multi-walled carbon nanotubes for selective catalytic reduction of NO with NH3: Catalytic activity and characterization. Chem Eng J. 2012;192:232–241.
50. Cristina ES, Wameedh A, Robbie B, et al. Ambient Temperature Hydrocarbon Selective Catalytic Reduction of NOx Using Atmospheric Pressure Nonthermal Plasma Activation of a Ag/Al2O3 Catalyst. ACS Catal. 2014;4:666–673.
51. Nguyen DB, Nguyen VT, Heo IJ, et al. Removal of NOx by Selective Catalytic Reduction Coupled with Plasma under Temperature Fluctuation Condition. J Ind Eng Chem. 2019;72:400–407.
52. Ölçenoğlu G, Saka C. Surface modification of coal sample with oxygen plasma treatment. Surf Eng. 2019;36:1–8.
53. Kondratowicz I, Nadolska M, Şahin S, et al. Tailoring properties of reduced graphene oxide by oxygen plasma treatment. Appl Surf Sci. 2018;440:651–659.
54. Zhao DS. Evaluation and characterization of catalysts. Beijing: Chemical Industry Press; 2011. p. 219–225.
55. Tang S, Lu N, Wang JK, et al. Novel effects of surface modification on activated carbon fibers using a low pressure plasma treatment. J Phys Chem C. 2007;111:1820–1829.
56. Chen C, Liang B, Ogino A, et al. Oxygen functionalization of multiwall carbon nanotubes by microwave-excited surface-wave plasma treatment. J Phys Chem C. 2009;113:7659–7665.
57. Saka C. Overview on the surface functionalization mechanism and determination of surface functional groups of plasma treated carbon nanotubes. Crit Rev Anal Chem. 2018;48:1–14.
58. Chambrion P, Orikasa H, Suzuki T, et al. A study of the C-NO reaction by using isotopically labelled C and NO. Fuel. 1997;76:493–498.
59. Chambrion P, Kyotani T, Tomita A. Role of N-containing surface species on NO reduction by carbon. Energ Fuel. 2012;12:416–421.
60. Ulán-Gómez MJ, Linares-Solano A, Radovic LR, et al. NO reduction by activated carbons. Some mechanistic aspects of uncatalyzed and catalyzed reaction. Coal Sci Technol. 1995;24:1799–1802.
61. Smith RN, Swinehart J, Lesnini D. The oxidation of cardon by nitric oxide. J Phys Chem. 1959;63:544–547.
62. Song C, Guo B, Sun XF, et al. Enrichment and degradation of tetracycline using three-dimensional graphene/MnO2 composites. Chem Eng J. 2019;358:1139–1146.
63. Zhao X, Niu C, Zhang L, et al. Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate. Chemosphere. 2018;204:11–21.
64. Wu M, Zhan W, Guo Y, et al. An effective Mn-Co mixed oxide catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen. Appl Catal A General. 2016;523:97–106.
65. Ma CY, Mu Z, Li JJ, et al. Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J Am Chem Soc. 2010;132:2608–2613.
66. An J, Lou J, Meng Q, et al. Non-thermal plasma injection-CeO2-WO3/TiO2 catalytic method for high-efficiency oxidation of elemental mercury in coal-fired flue gas. Chem Eng J. 2017;325:708–714.
67. López D, Calo J. The NO-carbon reaction: the influence of potassium and CO on reactivity and populations of oxygen surface complexes. Energ Fuel. 2007;21:1872–1877.
68. Wang T, Sun BM, Xiao HP, et al. Effect of water vapor on NO removal in a DBD reactor at different temperatures. Plasma Chem Plasma P. 2013;33:681–690.
69. Shi YR, Jiang ZX, Wu TL, et al. Study on the water vapor adsorption capacities of activated carbons. Chem Ind Forest Prod. 1982;2–12.
Fig. 2(a) NO conversion and (b) N2 selectivity under different SIE over the single DBD, DBD-FCRB and DBD-Mn/FCRB systems (428 mg/m3 NO, 8% O2, Ar used as the balance gas). 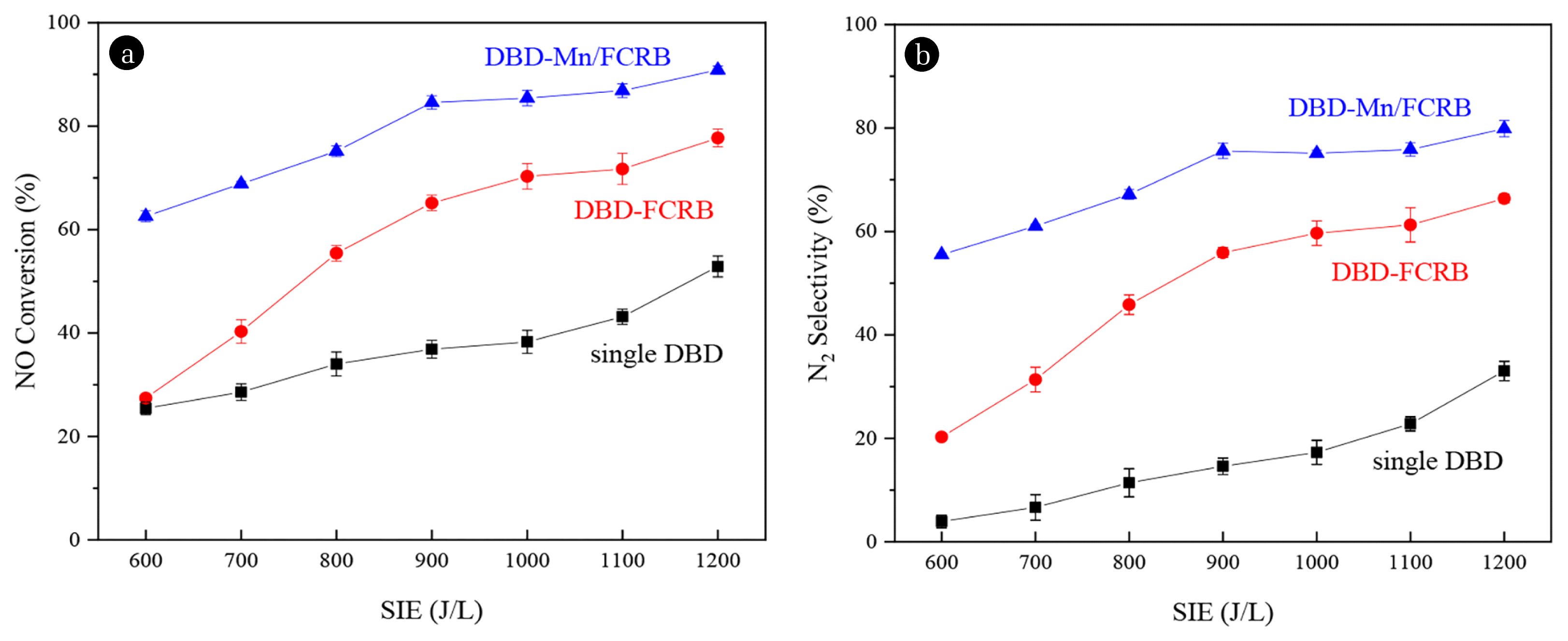
Fig. 3SEM images of FCRB: (a) blank FCRB, (b) the FCRB activated by DBD, and (c) the Mn/FCRB activated by DBD. 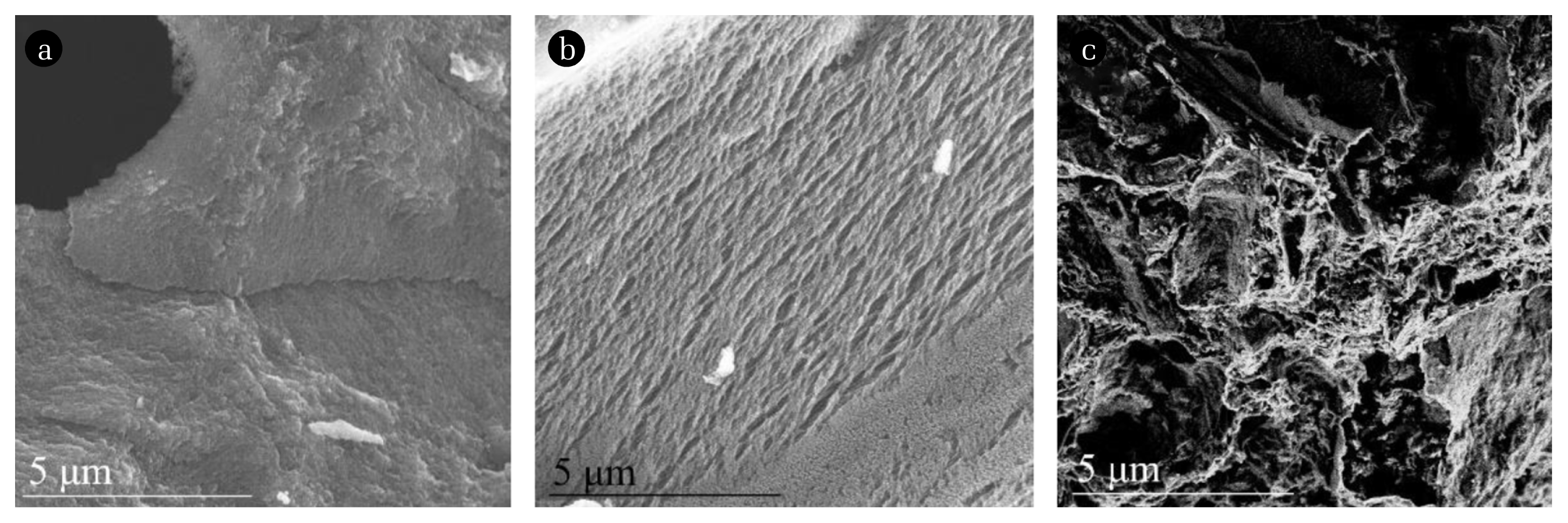
Fig. 4(a) XPS survey spectra of FCRBs, (b) XPS spectrum of C1s peak of B-FCRB and A-FCRB, and XPS spectrum of (c) Mn2p peak and (d) O1s peak of B-Mn/FCRB and A-Mn/FCRB. 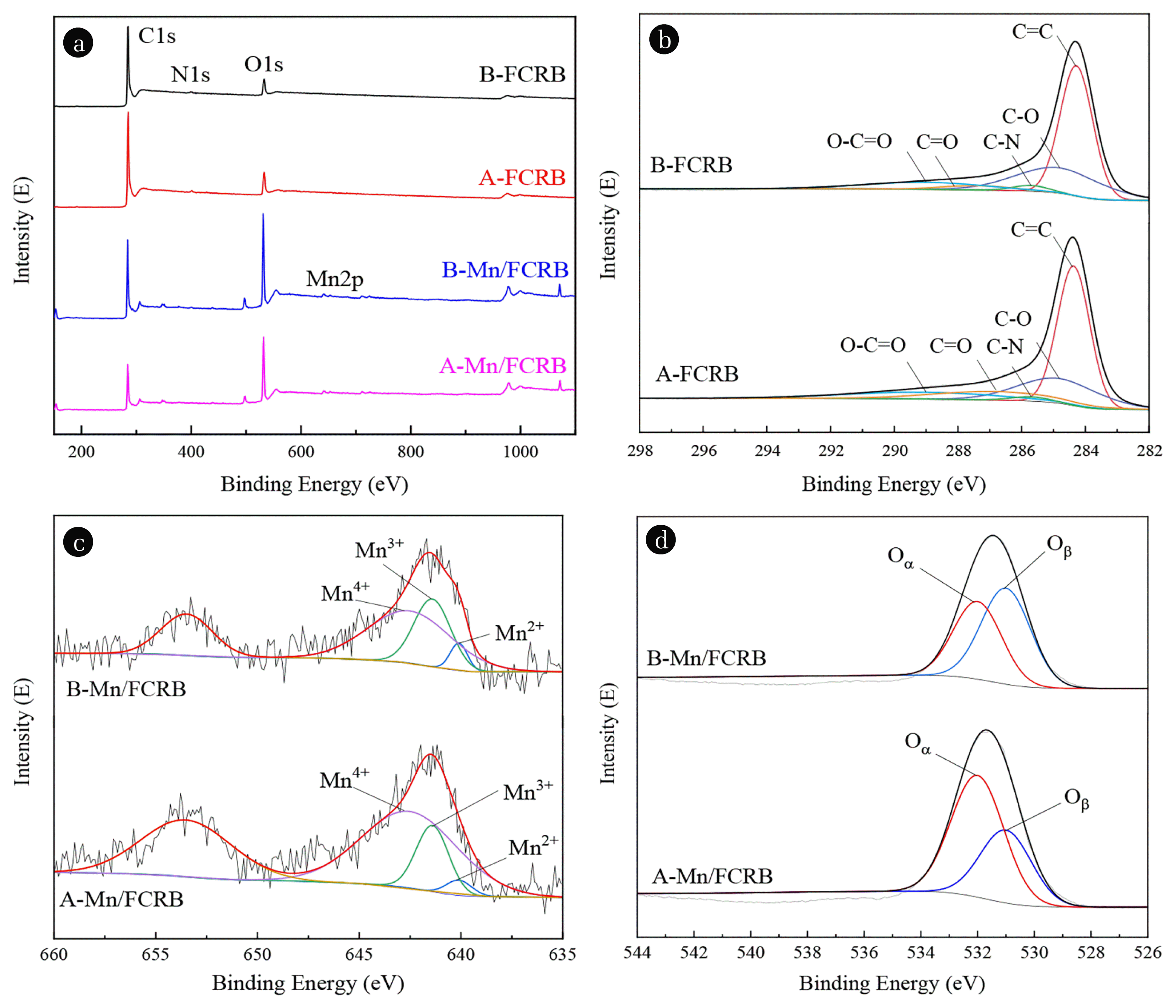
Fig. 5(a) GC spectrum observed with and without 428 mg/m3 NO in Ar and 0% O2 condition over the DBD-FCRB system (SIE = 1,000 J/L), and (b) CO and (c) CO2 concentration under different SIE in Ar and 8% O2 over the DBD-FCRB and DBD-Mn/FCRB system with and without 428 mg/m3 NO. 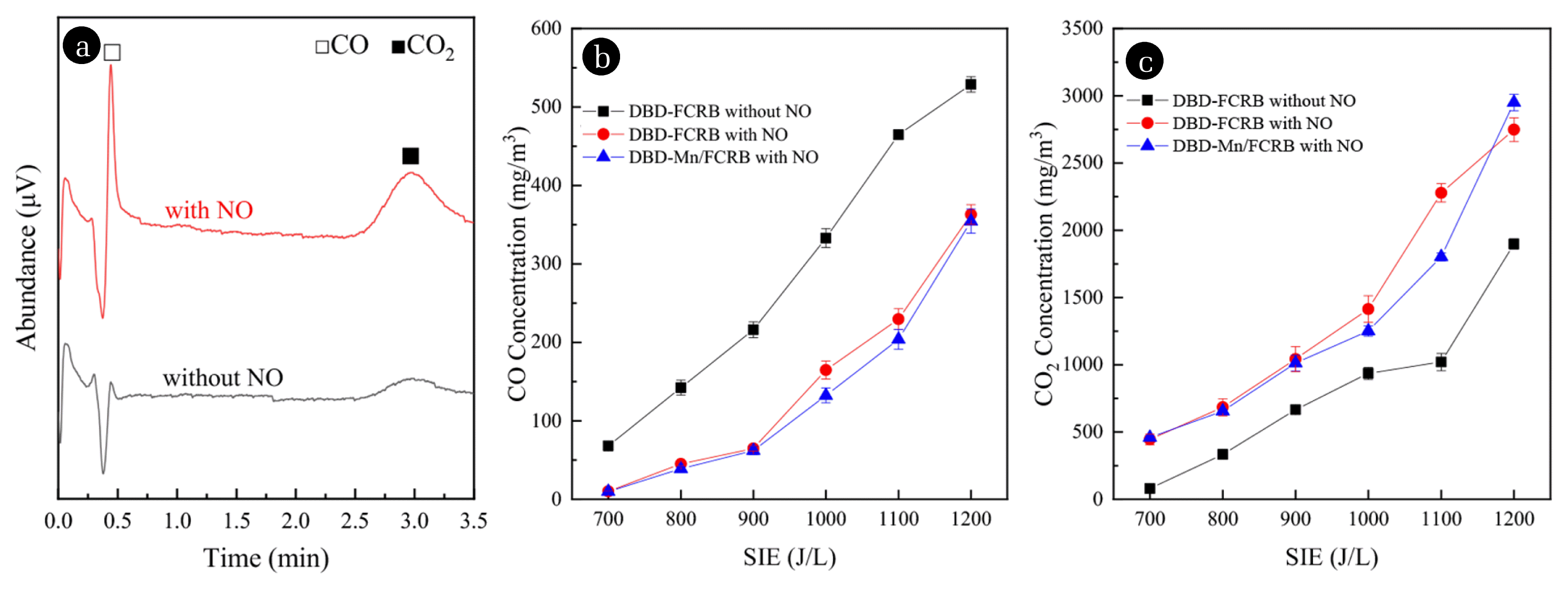
Fig. 6Effects of (a) O2 concentration and (b) H2O on NO conversion over the single DBD, DBD-FCRB, and DBD-Mn/FCRB systems (428 mg/m3 NO, Ar used as the balance gas, SIE = 800 J/L) 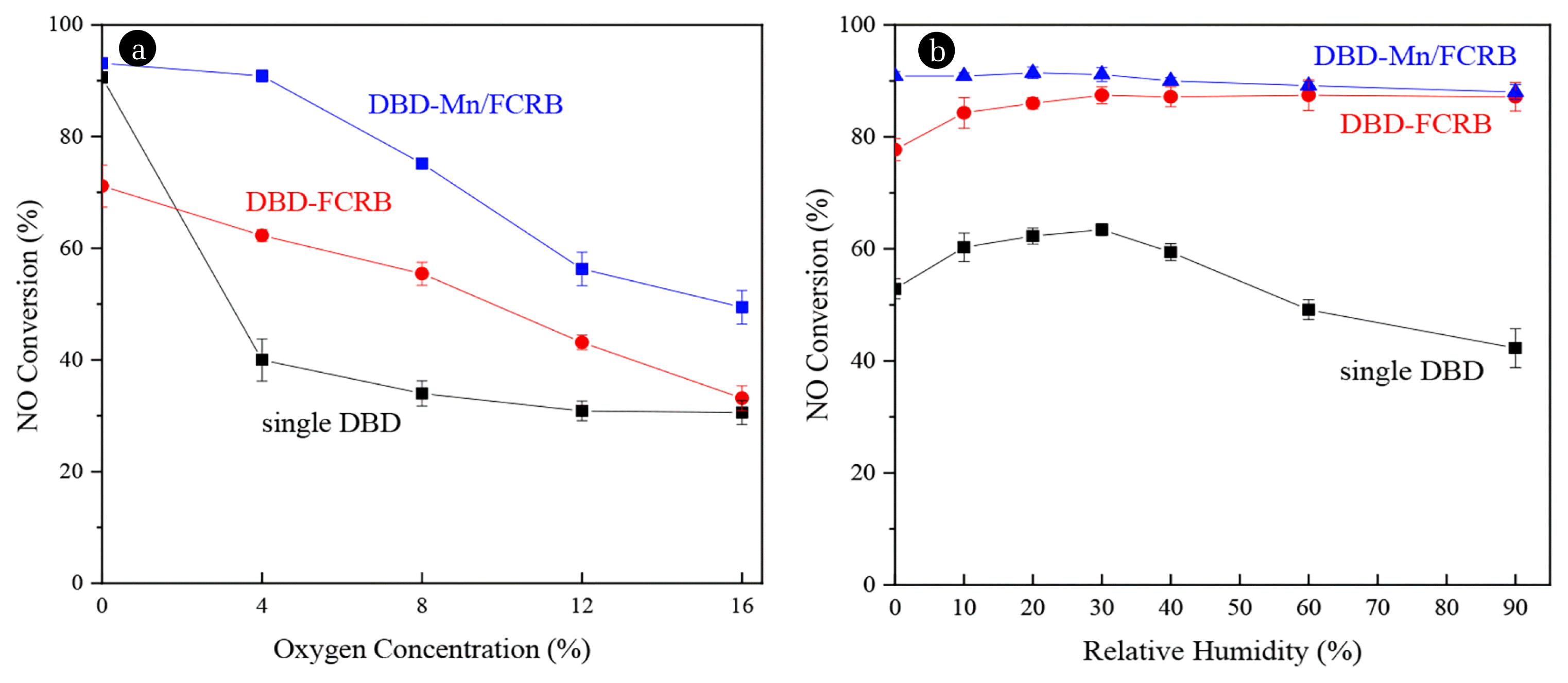
Table 1The Relative Content of Various Elements and Functional Groups Based on the XPS Spectra |
|
||||||||||||||||||||||||||||||||||||