AbstractThis investigation enables amino-functionalized metal–organic frameworks (MOFs) materials for the removal of imidacloprid (IMC). Two Fe-based MOF materials of NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) both exhibited high adsorption capacity and Fenton-like degradation ability for IMC which were utilized to remove IMC from aqueous solution. Although the adsorption capacity of NH2-MIL-101(Fe) was higher than that of NH2-MIL-88(Fe), the degradation abilities of both MOF materials were similar. The removal efficiencies were evaluated through several basic studies, including concentrations of catalyst (0.12–0.3 g/L) and IMC (20–100 mg/L), pH of solution (3–11), and amounts of 30% H2O2 (0–2.0 μL/mL). By optimizing the above factors, the total removal ratio of IMC by NH2-MIL-88B(Fe) was as high as 93%, whereas the removal ratio of NH2-MIL-101(Fe) was 97%. Moreover, these MOF materials were proven to be stable and recyclable. The free radical quenching experiment and density functional theory calculation were applied to research the removal mechanism, and the hydroxyl radicals (·OH) was found to be the key active intermediate. The high catalytic efficiency can be attributed to the synergy of the Fe3+/Fe2+ redox cycle.
1. IntroductionPesticides are used for controlling pests and regulating plant growth [1]. The neonicotinoid pesticide imidacloprid (IMC) is widely used in agriculture for pest control [2]. IMC are stable in nature and are difficult to degrade in sunlight. However, they are potentially dangerous to the environment, animals and human beings [3]. Residues of neonicotinoids have a profound impact on ecosystems, these include impacts on the water environment, the number and species of water animals, and the population level and health [4]. The widespread use of neonicotinoids in fruit and vegetable crops as well as their widespread presence in food has led to the production of excessive behaviors, such as those observed by Chen et al. [5] in which IMC has the highest detection ratio, and the detection values of snake fruit and green pepper exceeded the standard limitation. Currently, the impact of long-term, low-dose exposure on human health is not fully understood. Therefore, an effective treatment method must be established.
There are numerous methods for the removal of IMC, including adsorption[6], microbial decomposition [7], ozonation [8], electrochemistry [9], photocatalysis [10, 11] and advanced oxidation processes (AOPs) [12]. In these methods, AOPs is the most effective method which has been widely studied for its high efficiency, standardization and non-selectivity. Moreover, the Fenton process has attracted considerable attention in AOPs [13]. However, in practical application, the traditional Fenton technology has several disadvantages, such as high acidic requirements (2.8–3.5) [14], loss of catalyst, and low catalyst recovery rate. For example, Zouanti et al. [15] used Fenton and Fenton-like principles to removal oxytetracycline. They used ferrous sulfate heptahydrate and ferric sulfate as sources of Fe2+ and Fe3+. The degradation rate is fast after adding hydrogen peroxide (H2O2), however, the Fe source cannot be reused, and the pH requirement is strict (pH = 3–4). To solve these problems, researchers have developed and applied a heterogeneous catalyst [16].
Heterogeneous catalysts include catalysts containing Fe, copper, precious metals and rare earth elements. Among them, metal–organic frameworks (MOFs) have been used as heterogeneous catalysts. MOFs are a new type of catalyst with high catalytic activities [17], which have a porous structure, large size, different ligands for functionalization, and widely applications in many areas [18–20]. Among the MOFs catalysts, the Fe-based MOF as a Fenton-like catalyst has its advantages [17], this is because Fe has a wide source and is non-toxic [21]. Lv et al. [22] prepared a new type of FeII@MIL-100(Fe) catalyst for methylene blue degradation with high catalytic activity which is due to the synergistic effect of Fe (II) and Fe (III) ions in the production of ·OH. Li et al. [23] successfully synthesized a Fe (II) MOF material using 2,2′-bipyridine-5,5′-dicarboxylate as a ligand, which exhibited high catalytic activity for H2O2 under neutral conditions. Ma et al. [24] used NH2-MIL-88B to remove pefloxacin from aqueous solutions via adsorption and Fenton-like oxidation. However, to the best of our knowledge, no study has been reported on Fe-based MOF materials for the heterogeneous Fenton-like degradation of IMC. The Fe-based MIL material is very attractive and can act as a Fenton-like catalyst with high chemical and water stability [25]. In addition, the amine-functionalized MOF exhibits catalytic activity due to the electron transfer with the target [26]. It provides a heterogeneous catalyst with high activity, recycling utilization and good treatment effect for the actual treatment of wastewater. This is the first time to compare the degradation of IMC by two Fe-based MOF materials with NH2 groups.
Therefore, this study aimed to determine how amine-functionalized Fe-based MIL removes IMC via adsorption and Fenton-like reactions. NH2-MIL-101(Fe) and NH2-MIL-88(Fe) materials were synthesized using the hydrothermal method, and the adsorption equilibrium time was determined. Then, the Fenton-like degradation performances of NH2-Fe-based MOFs on IMC were compared. To obtain more application information, the influences of some key factors, such as the concentrations of catalyst and IMC, pH of the solution, and amount of H2O2 were investigated. The results can expand the application fields of amine-functionalized Fe-based MOF materials and elucidates how amine-functionalized Fe-based MIL can achieve the removal of IMC, thus providing more ideas for the future removal of pesticides.
2. Materials and Methods2.1. Chemicals and ReagentsIMC with 98% purity was obtained from Jianglai Biological Technology Co., Ltd. (Shanghai, China). Iron trichloride hexahydrate (FeCl3·6H2O) and 2-aminoterephthalic acid (NH2-BDC) were purchased from Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). N, N′-dimethylformamide (DMF), ethanol (EtOH), 1,4-benzoquinone (BQ), and H2O2 (30%) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All of the solutions were prepared using ultrapure water. Acetonitrile and methanol (chromatographic grade) were obtained from TEDIA (Anhui, China).
2.2. Characterizations of NH2-Fe-MILsX-ray powder diffraction (XRD) patterns were investigated on a BRUKER AXS D8-Advance X-ray system. X-ray photoelectron spectroscopy (XPS) analysis was conducted on a Thermo Scientific K-Alpha. Fourier-transform infrared (FTIR) spectra were recorded as KBr disks and mulls in Nujol using a Nicolet FTIR 5700 spectrometer. The morphology and crystal size of the particles were characterized by using a scanning electron microscope (SEM, QUANTA F250). Thermogravimetric analysis (TGA) was characterized using an SDT Q600 thermal analyzer under an airflow of 100 mL/min at a heating rate of 10°C/min.
2.3. Adsorption ExperimentsFirst, the two reactors were filled with 50 mL of IMC solution (50 mg/L), and then, 10 mg of synthetic NH2-MIL-88B(Fe) or NH2-MIL-101(Fe) was added to the reactors. Subsequently, the reaction system was placed under a magnetic stirrer and then evenly stirred. The experiment time was set to 300 min. At certain intervals, 1.0 mL of the sample was drawn using a 1-mL syringe and filtered with a 0.22-μm polytetrafluoroethylene syringe filter. The filtered sample was detected using the Waters e2695 high-performance liquid chromatography (HPLC) system with a C18 column (4.6 × 250 mm, particle size 5 μm). The detection conditions were water/acetonitrile (70:30, v/v) at a flow rate of 1.0 mL/min, a column temperature of 30°C, and UV detector operated with 270 nm.
2.4. Degradation ExperimentsBy adding H2O2 after the adsorption process, the Fenton-like degradation activity of NH2-Fe-MILs was achieved. 10 mg NH2-Fe-MILs catalyst were added into 50 mL IMC aqueous solution (50 mg/L), then, to achieve the adsorption–desorption equilibrium between the catalyst and IMC molecules, the mixed solution was stirred for 60 min under magnetic stirring, and 1.0 mL of the solution was filtered to measure the concentration of IMC after adsorption. After adsorption, we added an appropriate amount of 30% H2O2 to the reactor, and the duration of the degradation process was 90 min. Then, the IMC concentration after degradation was measured. Moreover, the reusability of the catalyst was tested. After each cycle, ethanol was used to remove the contaminants remaining on the catalyst and then vacuum-dried at 70°C overnight to prepare for the next cycle [27]. The oxidation products of IMC were also investigated by The ACQUITYTM ultra performance liquid chromatography and Waters XevoTM TQD mass spectrometer (UPLC–MS/MS, Waters Co., USA). Chromatographic separation was performed on the AcquityTM UPLC system with a Waters ACQUITY UPLC HILIC chromatographic column (50 mm × 2.1 mm, 1.7 μm). Separation of the sample was achieved by an isocratic elution program with the mobile phase of a mixture of acetonitrile and ultrapure water (V:V = 50:50). Column temperature was set as 35°C. The flow rate and injection volume were 0.3 mL/min and 5.0 μL, respectively. The electro-spray ionization source (ESI) was operated using the positive mode with capillary voltage of 3.5 kV and the ion source temperature of 150°C. The MS detector was performed in mass scan mode. The desolvation temperature of 450°C was used and the flow rates of the desolvation gas, cone gas and collision gas (Ar) were set at 800 L/h, 50 L/h and 0.20 mL/min respectively. The mass spectra were collected at a range of 100–300 m/z.
2.5. Calculation DetailsAll the calculations in this work were performed by using the DMol3 software package [28] based on density functional theory (DFT) [29]. The PW91 exchange–correlation functional was adopted for generalized gradient approximation (GGA) correction. All-electron Kohn–Sham wave functions were expanded in a double numerical plus d-functions (DND) basis. The sampling of irreducible wedge of Brillouin zone was performed with a regular Monkhorst–Pack grid of special k-points. The convergences criteria of relaxation were 2.0 × 10−5 Ha, 0.004 Ha/Å and 0.009 Å for energy, gradient and atomic displacement, respectively.
3. Results and Discussion3.1. Characterizations of NH2-Fe-MILsNH2-Fe-MILs were synthesized according the reports and the details were shown in supporting materials. FTIR studies (Fig. 1) were conducted to investigate the molecular structure and functional groups of prepared materials. In the spectrum of NH2-MIL-88B(Fe), the peaks at 3,463 and 3,342 cm−1 were attributed to the symmetrical and asymmetrical vibrations of the −NH2 group [30]. In addition, the peaks at 1,384 and 1,578 cm−1 were attributed to the carboxyl vibration [31]. A tiny peak at 1,257 cm−1 indicated the C–N stretching [32]. The peak at 769 cm−1 indicated the C–H bending vibration of the benzene ring [27]. And the peak observed at 588 cm−1 indicated the stretching vibration of the Fe–O bond in NH2-MIL-88B(Fe) [33]. As can be seen from Fig. 1, the FTIR spectra of NH2-MIL-101(Fe) and NH2-MIL-88B(Fe) are similar. The XRD patterns of the two MOFs (Fig. 2) indicate the typical main diffraction peaks of the structure and are consistent with the data presented in the literatures [34,35]. The characteristic diffraction peaks of the as-prepared NH2-MIL-88B(Fe) appeared at approximately 9.2°, 10.2°, 16.5°, 18.7°, 19.3°, 20.5°, 25.7° and 28.1°, which correspond to the Miller exponents (002), (101), (102), (103), (200), (202), (204) and (302) on the simulated NH2-MIL-88B(Fe) [36]. The simulated MIL-101 (Fe) has high intensity diffraction peaks on Miller index (511), (531), (852), (753), (1022) and (880), while the synthesized NH2-MIL-101 (Fe) has similar peaks and higher intensity, indicating that the introduction of amino ligands disrupted the structure of MIL-101 (Fe) to a certain extent [34].
The morphology and element content of the NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) materials were observed via SEM and energy-dispersive spectroscopy (EDS). The SEM images of NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) are presented in Fig. S1. The morphology of NH2-MIL-88B(Fe) is a hexagonal micro-spindle crystal with a length of about 1 μm and a width of about 400 nm, similar to the length and width reported in the previous literature [37]. NH2-MIL-101(Fe) exhibits a regular and well-defined octahedral structure with a particle size ranging from 500 nm to 1 μm. Fig. S1(c) and (d) present the composition analysis of NH2-Fe-MILs samples measured via EDS. The percentages of the Fe, O, C, and N elements were obtained as 21.12%, 16.06%, 48.65%, and 9.78% for NH2-MIL-88B(Fe), respectively, and 21.60%, 16.78%, 45.83%, and 9.29% for NH2-MIL-101(Fe), respectively.
The thermal stabilities of the NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) samples were evaluated via TG analysis. As presented in Fig. S2, the TG curves of the NH2-Fe-MILs samples remained stable until 250°C, a finding consistent with those of previous reports [2,38]. In the curves of NH2-MIL-88B(Fe), the first weight loss (12.5%) occurred between 25°C and 250°C may correspond to the loss of water and DMF present in the materials. Compared with NH2-MIL-88B(Fe), NH2-MIL-101(Fe) exhibited a greater mass loss at this stage, probably because NH2-MIL-101(Fe) adsorbed more solvent molecules. The second loss may be caused by the free carboxyl groups in NH2-Fe-MILs, whereas the third loss may be due to the partial skeleton decomposition of NH2-Fe-MILs and the low residual amount of NH2-MIL-101(Fe).
The chemical composition and oxidation state of the sample were analyzed via XPS characterization. The NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) materials constitute four elements (C 1s, N 1s, O 1s, and Fe 2p) (Fig. S3). For the XPS spectrum of C 1s (Fig. S4), NH2-MIL-88B(Fe) has main lines with binding energies of 284.8, 285.3, and 288.8 eV. The characteristic peak at 284.8 eV can be attributed to the alkyl carbon component of the benzene ring. Moreover, 285.3 and 288.8 eV may be strongly associated with Csp2-N of aromatic C coupling with amino NH2 and C=O groups of benzoic acid, respectively, which is in agreement with a previous report by Guo et al. [30]. The Fe 2p spectrum of NH2-MIL-88B(Fe) exhibits two main peaks and one satellite peak. The peak at 711.6 eV is attributed to Fe 2p3/2, the peak at 725.3 eV to Fe 2p1/2, and the peak at 713.7 eV to the satellite signal [39]. For the NH2-MIL-101(Fe), the spectra of C 1s binding energies were observed at 284.8, 286.6, and 289.3 eV. Conversely, the spectra of Fe 2p binding energies were observed at 711.9, 714.9, and 726.0 eV. In NH2-Fe-MILs, the binding energy position of Fe 2p1/2 and Fe 2p3/2 peak separation (Δ = 2p1/2 – 2p3/2) were 13.7 and 14.1 eV, respectively, which is similar to the reported Fe2O3 [40].
3.2. IMC Removal Performances of NH2-Fe-MILsBefore the Fenton-like degradation experiments, adsorption experiments are required to determine the adsorption equilibrium time. As presented in Fig. S5(a), for NH2-MIL-101(Fe), the reaction reaches the adsorption equilibrium within 20 min, whereas for NH2-MIL-88B(Fe), the reaction reaches the adsorption equilibrium after 60 min. Accordingly, before degradation experiments were conducted, it was necessary to perform adsorption for 60 min. To compare the adsorption and degradation of IMC, the degradation experiment of IMC was conducted under the same conditions after adsorption for 60 min. The C/C0 was used to describe the adsorption and degradation processes. C denotes the IMC concentration at time t, whereas C0 denotes the initial concentration of IMC. As presented in Fig. S5(b), for NH2-MIL-101(Fe), after adsorbing 70.6% of IMC, at least 17.75% of IMC was removed during the Fenton-like degradation process, thus reaching 88.35% in total, whereas NH2-MIL-101(Fe) exhibited higher activity than that of NH2-MIL-88(Fe) (79.9%).
3.3. Adsorption Mechanism of NH2-Fe-MILs3.3.1. Adsorption modesTo understand the adsorption process and the material adsorption capacity for IMC, the adsorption isotherm experiment of IMC on NH2-Fe-MILs was conducted at room temperature at five initial concentrations: 10, 30, 50, 70, and 90 mg/L [41]. As can be seen from Fig. S6(a) and (b), the adsorption of IMC on NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) both fit the Langmuir model more than the Freundlich model (Eq. (S1) and (S2) [42–44]), indicating that the NH2-Fe-MILs have uniformly distributed functional groups on the surface, and the adsorption process of IMC is surface monolayer adsorption.
To further study the interactions between NH2-Fe-MILs and IMC, the experimental results were fitted to a kinetic model (Eq. (S3) and (S4) [42, 45]). Fig. S6(c) and (d) present the results of kinetic models. NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) both fit well with the second-order model. From this result, we can infer the adsorption process, initially, the second-order model may indicate that the IMC molecules may diffuse to the surface of NH2-Fe-MILs firstly and then diffuse into the pores.
3.3.2. The influence of different solution pH on the adsorption of IMCThe pH value of the solution directly influences the stability of the MOF, the surface potential, and the degree of IMC ionization. Therefore, we investigated the effect of solution pH on the adsorption rate during the adsorption of IMC as well as the surface potential of MOFs under different pH conditions.
In the pH range of 3–11, the effect of pH on the adsorption capacity of NH2-Fe-MILs was estimated (Fig. S7(a)). It can be seen that when the pH is from 3–9, the two materials exhibit high adsorption rate, however, when the pH is 11, the adsorption rate sharply decreases. The effect of pH on adsorption behavior of IMC can be explained based on the point of zero charge (pHzpc). As presented in Fig. S7(b), the pHzpc value of the NH2-MIL-88B(Fe) and NH2-MIL-101 (Fe) are 6.7 and 9.3, respectively. The IMC molecules exhibit the aqueous dissociation constants as pK1 = 2.3, pK2 = 5.7, and pK3 = 11. Hence, in most of the experimental pH range, IMC is negatively charged. We found that the surface charge of NH2-MIL-88B(Fe) remained negative above pH 6.7 and that NH2-MIL-101(Fe) is negatively charged above pH 9.3 from the principle of electrostatic adsorption. However, NH2-Fe-MILs have higher adsorption capacity in 3–9, which may be due to the electrostatic adsorption and chemical adsorption between imidacloprid molecules and NH2-Fe-MILs. This idea is also consistent with the previous results.
3.4. Degradation of IMC3.4.1. Effect of processing parameters on the removal efficiencyThe above experiment explored the ability of NH2-Fe-MILs to remove IMC. In order to optimize the removal effect, the effects of the initial concentration of catalyst, initial concentration of IMC, pH and concentration of H2O2 on IMC removal were studied. For the removal experiment, the effects of the NH2-Fe-MILs doses (0.12–0.36 g/L) on the removal of IMC were evaluated. As presented in Fig. 3(a) and 4(a), the adsorption capacity of NH2-MIL-101(Fe) was stronger than that of NH2-MIL-88B(Fe) and that the removal efficiency of IMC increased with the addition of more doses. The removal ratio of NH2-MIL-88B(Fe) increased from 41.20% to 87.98%, and that of NH2-MIL-101(Fe) increased from 67.36% to 96.89%. This enhanced the removal efficiency and could be attributed to the NH2-Fe-MILs heterogeneous catalysts having a large specific surface area and the ability to generate ·OH via the decomposition of H2O2. However, after considering the removal efficiency and economy, 0.3 g/L of NH2-MIL-88(Fe) and 0.24 g/L of NH2-MIL-101(Fe) were chosen as the best dosage in the subsequent experiments.
For wastewater treatment, it is important to investigate the influence of the initial pollutant concentration [27]. The study was conducted at an initial concentration of 20 to 100 mg/L, and the results are presented in Fig. 3(b) and 4(b). The increase in the initial IMC concentration was not conducive to the IMC removal. Moreover, the removal efficiency of NH2-MIL-88B(Fe) decreased from 97.76% (C0 = 20 mg/L) to 74.59% (C0 = 100 mg/L), whereas the removal efficiency of NH2-MIL-101(Fe) decreased from 97.03% to 84.69%. Notably, compared with the NH2-MIL-88B(Fe) material, the NH2-MIL-101(Fe) material exhibited higher activity in the adsorption and Fenton-like degradation of IMC at a relatively lower dose. Here, we chose a 60 mg/L IMC solution for the next experiment.
In a typical Fenton-like reaction, H2O2 is utilized to produce hydroxyl radicals (·OH). As presented in Fig. 3(c) and 4(c), when the catalyst reached the adsorption equilibrium, without the addition of H2O2, the removal rate of IMC did not continue to decrease. However, when the dosage of H2O2 was increased, the removal ratio of IMC continued to increase.
The effect of initial pH 3–11 on the IMC removal performance of the MOF-based catalyst is shown in Fig. 3(d) and 4(d). When the pH value is 11, the removal efficiency of NH2-Fe-MILs decreases, and when the pH range is (3–9), both have a good removal effect. The experimental results are consistent with many works, and the removal effect of NH2-Fe-MILs can play a role in a wide pH range. The high removal efficiency at a low pH can be explained by the following: the Fenton-like reaction between H2O2 and Fe in the catalyst is more likely to occur in an acidic environment [22]. The results indicate the ability to overcome the drawback of a strict pH regulation (pH 2.8–3.5) for the traditional Fenton process [14]. In general, the best reaction parameters were 0.3 g/L of NH2-MIL-88B(Fe) or 0.24 g/L of NH2-MIL-101(Fe), IMC concentration is 60 mg/L, pH is 7 and 2.0 μL/mL of 30% H2O2.
3.4.2. Degradation mechanismThe generation of ·OH is due to Fe2+ catalyzing the decomposition of H2O2 [46], the formed ferric ions (Fe3+) can be reduced using Eq. (1) and (2). In order to prove that hydroxyl radicals were generated in the reaction system, ·OH and ·O2 − trapping experiments were carried out. First, two different scavengers, namely, BQ (·O2 − scavenger) and EtOH (·OH scavenger), were put into the present system. As demonstrated in Fig. 5, the reaction rate decreased after the addition of the free radical scavenger especially the addition of EtOH. Compared with the blank without scavenger, it can be concluded that ·OH is the main active substances causing the degradation of IMC. In order to further confirm the main active substances produced in the degradation process, DFT calculations are first used to illustrate the reaction process of Fenton-like reaction catalyzed by MIL-88B(Fe). The model is based on the local structural unit of MIL-88B(Fe) containing three Fe atoms, and the upper exposed Fe is the reaction site (*). First, the adsorbed H2O2* is sheared into ·OH and adsorbed hydroxyl (HO*), and then the protonated hydrogen is close to ·OH to form H2O. It can be seen from Fig. 6 that the adsorption of H2O2* is thermodynamically favorable. The formation and desorption of H2O is also thermodynamically favorable, and once again proves that ·OH is produced theoretically.
3.4.3 XPS analysisIn order to explore the recognition mechanism between NH2-Fe-MILs and IMC, XPS spectra of NH2-Fe-MILs after adsorption and degradation of IMC were analyzed, respectively. The full-scan spectra of XPS (Fig. 7(a) and (b)) showed that the peak strength of C1s and O1s increased significantly after IMC was adsorbed, indicating that IMC was successfully adsorbed on NH2-Fe-MILs [45]. Therefore, the excellent adsorption of NH2-Fe-MILs is due to chemical adsorption. After Fenton-like reaction, the peak strength of C1s and O1s decreased slightly, indicating that IMC adsorbed on NH2-Fe-MILs was degraded. In addition, it is known from the above experimental results that Fe ions play a key role in the degradation reaction [47]. In order to confirm that the valence state of Fe in the catalyst changed before and after the Fenton-like degradation of IMC, XPS test was performed. As shown in Fig. 7(c) and (d), the BE value of Fe 2p3/2 of the NH2-Fe-MILs material after the Fenton-like reaction is lower than the BE value before the reaction, which can be attributed to the conversion of Fe (III) to Fe (II) after the Fenton-like reaction [48].
3.4.4. Reusability of NH2-Fe-MILsReusability is an important indicator of the practicality of solid catalysts. To investigate the reusability of these two catalysts, the NH2-Fe-MILs catalysts were collected by washing and centrifugation after each cycle of process. As presented in Fig. S8, the removal efficiencies of the two catalysts are only slightly reduced after regenerations, indicating that the regenerations of the catalyst were practicable. Moreover, Fig. S9 demonstrates that the FT-IR spectrum of the materials has no significant change. This means that there is no significant difference between the chemical bond and crystal structure of the sample before and after the reaction. Therefore, the NH2-Fe-MILs material has a strong reusability performance. Table S1 clearly shows that NH2-Fe-MILs material is an effective heterogeneous catalyst for the removal of IMC from water which means it has the potential ability to remove other organic pollutants in water.
3.5. Analysis of Oxidation ProductsTo confirm that IMC was oxidized, we employed UPLC–MS/MS to determine the final degraded substances, and the results are presented in Fig. S10. The total ion current demonstrated that IMC after the degradation produced small species. The MS2 spectrum presented in Fig. 8(a) shows detailed information of each intermediate segment, we can see the molecular ion peak of imidacloprid parent ion at m/z 256, and the main fragments appearing are m/z 300 (two Na added), m/z 278 (one Na added), m/z 209 (NO2 lost) and m/z 175 (NO2 and Cl lost). Fig. 8(b) shows the molecular ion peaks of the degradation intermediates, the predominant intermediates identified for IMC were compounds with m/z 129, 187, 229 and 269. Fig. S11 hints at the possible structure of these molecular ion peaks, and infers that the main degradation intermediate is the substance with m/z 269 (C9H8ClN5O3), which was the oxidation product of IMC.
4. ConclusionsIn this study, NH2-Fe-MILs were successfully prepared by hydrothermal synthesis, which are effective adsorbents and Fenton-like catalysts. According to the experimental results, the differences between NH2-MIL-88B(Fe) and NH2-MIL-101(Fe) are that the latter has a smaller particle size and a larger area of contact with the target IMC. In addition, the two MOFs can effectively remove IMC by adsorption and degradation over a wide pH range (3–9). Furthermore, free radical scavenging experiments and DFT calculation both revealed that the main active substance of ·OH was generated in the Fenton-like reaction to degrade IMC. Finally, based on the results of LC-MS, possible degradation intermediates of IMC are proposed.
AcknowledgmentsThis research was funded by the National Natural Science Foundation of China (31601550) and Natural Science Foundation of Hunan (2019JJ50638).
NotesAuthor Contributions M.C. (Associate Professor) obtained funding, wrote and revised the manuscript. T.L. (Master Student) conducted the experiments and wrote the manuscript. L.L. (Master Student) conducted the theory calculations. Z.X. (Associate Professor) revised the manuscript. L.D. (Professor) revised the manuscript. Y.C. (Professor) provided resources, wrote and revised the manuscript. References1. Amalraj A, Pius A. Photocatalytic degradation of monocrotophos and chlorpyrifos in aqueous solution using TiO2 under UV radiation. J Water Process Eng. 2015;7:94–101.
2. Gecgel C, Simsek UB, Gozmen B, Turabik M. Comparison of MIL-101(Fe) and amine-functionalized MIL-101(Fe) as photocatalysts for the removal of imidacloprid in aqueous solution. J Iran Chem Soc. 2019;16:1735–1748.
3. Bovi TS, Zaluski R, Orsi RO. Toxicity and motor changes in Africanized honey bees (Apis mellifera L.) exposed to fipronil and imidacloprid. An Acad Bras Ciênc. 2018;90:239–245.
4. Sánchez-Bayo F, Goka K, Hayasaka D. Contamination of the aquatic environment with neonicotinoids and its implication for ecosystems. Front Environ Sci. 2016;471(1–14)
5. Chen M, Tao L, McLean J, Lu CS. Quantitative analysis of neonicotinoid insecticide residues in foods: Implication for dietary exposures. J Agric Food Chem. 2014;62:6082–6090.
6. Liu GY, Li LY, Xu DH, et al. Metal-organic framework preparation using magnetic graphene oxide-beta-cyclodextrin for neonicotinoid pesticide adsorption and removal. Carbohydr Polym. 2017;175:584–591.
7. Sharma T, Kaur M, Sobti A, Rajor A, Toor AP. Sequential microbial-photocatalytic degradation of imidacloprid. Environ Eng Res. 2020;25:597–604.
8. King JF, Szczuka A, Zhang Z, Mitch WA. Efficacy of ozone for removal of pesticides, metals and indicator virus from reverse osmosis concentrates generated during potable reuse of municipal wastewaters. Water Res. 2020;176:115744.
9. Tawade AK, Kumar DM, Talele P, Sharma KKK, Tayade SN. Flower-Like ZnO-Decorated Polyaniline-Graphene oxide nanocomposite for electrochemical oxidation of imidacloprid: A hybrid nanocomposite sensor. J Electron Mater. 2019;48:7747–7755.
10. Heng HM, Yang JP, Yin YJ, Meng PC, Liu X. Effect of precursor types on the performance of polyimide: A metal-free visible-light-driven photocatalyst for effective photocatalytic degradation of pollutants. Catal Today. 2020;340:225–235.
11. Ahmari H, Heris SZ, Khayyat MH. The effect of titanium dioxide nanoparticles and UV irradiation on photocatalytic degradation of Imidaclopride. Environ Technol. 2018;39:536–547.
12. Gonzalez T, Dominguez JR, Correia S. Neonicotinoids removal by associated binary, tertiary and quaternary advanced oxidation processes: Synergistic effects, kinetics and mineralization. J Environ Manag. 2020;261:110156.
13. Huang DL, Hu CJ, Zeng GM, et al. Combination of Fenton processes and biotreatment for wastewater treatment and soil remediation. Sci Total Environ. 2017;574:1599–1610.
14. Katsumata H, Kaneco S, Suzuki T, Ohta K, Yobiko Y. Degradation of linuron in aqueous solution by the photo-Fenton reaction. Chem Eng J. 2005;108:269–276.
15. Zouanti M, Bezzina M, Dhib R. Experimental study of degradation and biodegradability of oxytetracycline antibiotic in aqueous solution using Fenton process. Environ Eng Res. 2020;25:316–323.
16. Singh JW, Chang YY, Koduru JR, Yang JK. Potential degradation of methylene blue (MB) by nano-metallic particles: A kinetic study and possible mechanism of MB degradation. Environ Eng Res. 2018;23:1–9.
17. Cheng M, Lai C, Liu Y, et al. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord Chem Rev. 2018;368:80–92.
18. Xu Z, Long LL, Chen YQ, Chen ML, Cheng YH. A nanozyme-linked immunosorbent assay based on metal-organic frameworks (MOFs) for sensitive detection of aflatoxin B-1. Food Chem. 2021;338:128039.
19. Chen ML, Ning P, Jiao Y, Xu Z, Cheng YH. Extraction of antioxidant peptides from rice dreg protein hydrolysate via an angling method. Food Chem. 2021;337:128069.
20. Ning P, Cheng YH, Xu Z, Ding L, Chen ML. Application of Metal-organic framework materials in enrichment of active peptides. Prog Chem. 2020;32:497–504.
21. Wang D, Wang M, Li Z. Fe-based Metal–organic frameworks for highly selective photocatalytic benzene hydroxylation to phenol. ACS Catal. 2015;5:6852–6857.
22. Lv HL, Zhao HY, Cao TC, et al. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. J Mol Catal A Chem. 2015;400:81–89.
23. Li Y, Liu H, Li WJ, Zhao FY, Ruan WJ. A nanoscale Fe(II) metal-organic framework with a bipyridinedicarboxylate ligand as a high performance heterogeneous Fenton catalyst. Rsc Advances. 2016;6:6756–6760.
24. Ma H, Yu B, Wang Q, Owens G, Chen Z. Enhanced removal of pefloxacin from aqueous solution by adsorption and Fenton-like oxidation using NH2-MIL-88B. J Colloid Interface Sci. 2021;583:279–287.
25. Liang RW, Chen R, Jing FF, Qin N, Wu L. Multifunctional polyoxometalates encapsulated in MIL-100(Fe): highly efficient photocatalysts for selective transformation under visible light. Dalton Trans. 2015;44:18227–18236.
26. Emerson AJ, Chahine A, Batten SR, Turner DR. Synthetic approaches for the incorporation of free amine functionalities in porous coordination polymers for enhanced CO2 sorption. Coord Chem Rev. 2018;365:1–22.
27. Wang DB, Jia FY, Wang H, et al. Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J Colloid Interface Sci. 2018;519:273–284.
28. Delley B. From molecules to solids with the DMol3 approach. J Chem Phys. 2000;113:7756–7764.
29. Delley B. DMol3 DFT studies: from molecules and molecular environments to surfaces and solids. Comput Mater Sci. 2000;17:122–126.
30. Guo HX, Niu BT, Wu XM, Zhang Y, Ying SM. Effective removal of 2,4,6-trinitrophenol over hexagonal metal-organic framework NH2-MIL-88B(Fe). Appl Organomet Chem. 2019;33:e4580.
31. Xie DH, Ma Y, Gu Y, et al. Bifunctional NH2-MIL-88(Fe) metal-organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J Mater Chem. 2017;5:23794–23804.
32. Yuan RR, Yue CL, Qiu JL, Liu FQ, Li AM. Highly efficient sunlight-driven reduction of Cr(VI) by TiO2@NH2-MIL-88B(Fe) heterostructures under neutral conditions. Appl Catal B Environ. 2019;251:229–239.
33. Hou SL, Wu YN, Feng LY, et al. Green synthesis and evaluation of an iron-based metal-organic framework MIL-88B for efficient decontamination of arsenate from water. Dalton Transactions. 2018;47:2222–2231.
34. Bauer S, Serre C, Devic T, et al. High-throughput assisted rationalization of the formation of metal organic frameworks in the Iron(III) aminoterephthalate solvothermal system. Inorg Chem. 2008;47:7568–76.
35. Tian N, Jia QM, Su HY, et al. The synthesis of mesostructured NH2-MIL-101(Cr) and kinetic and thermodynamic study in tetracycline aqueous solutions. J Porous Mater. 2016;23:1269–1278.
36. Shao L, Yu Z, Li X, et al. Carbon nanodots anchored onto the metal-organic framework NH2-MIL-88B(Fe) as a novel visible light-driven photocatalyst: Photocatalytic performance and mechanism investigation. Appl Surface Sci. 2020;505:144616.
37. Shi L, Wang T, Zhang HB, et al. An Amine-Functionalized Iron(III) Metal-Organic Framework as Efficient Visible-Light Photocatalyst for Cr(VI) Reduction. Adv science. 2015;2:1500006.
38. Ma MY, Noei H, Mienert B, et al. Iron metal organic frameworks MIL-88B and NH2-MIL-88B for the loading and delivery of the gasotransmitter carbon monoxide. Chem-Eur J. 2013;19:6785–6790.
39. Li YY, Jiang J, Fang Y, et al. TiO2 nanoparticles anchored onto the Metal-organic framework NH2-MIL-88B(Fe) as an adsorptive photocatalyst with enhanced Fenton-like degradation of organic pollutants under visible light irradiation. ACS Sustain Chem Eng. 2018;6:16186–16197.
40. Descostes M, Mercier F, Thromat N, Beaucaire C, Gautier-Soyer M. Use of XPS in the determination of chemical environment and oxidation state of iron and sulfur samples: constitution of a data basis in binding energies for Fe and S reference compounds and applications to the evidence of surface species of an oxidized pyrite in a carbonate medium. Appl Surf Sci. 2020;165:288–302.
41. Chen ML, Zhou SY, Xu Z, Ding L, Cheng YH. Metal-organic frameworks of MIL-100(Fe, Cr) and MIL-101(Cr) for aromatic amines adsorption from aqueous solutions. Molecules. 2019;24:3718.
42. Balati A, Tek S, Nash K, Shipley H. Nanoarchitecture of TiO2 microspheres with expanded lattice interlayers and its hetero-junction to the laser modified black TiO2 using pulsed laser ablation in liquid with improved photocatalytic performance under visible light irradiation. J Colloid Interface Sci. 2019;541:234–248.
43. Balati A, Ghanbari M, Behzad KS, Amini MM. Functionalization of graphene oxide with 9-aminoanthracene for the adsorptive removal of persistent aromatic pollutants from aqueous solution. Acta Chim Slovenica. 2017;64:479–490.
44. Hashemi SH, Amini MM, Shahbazi A, Balati A. Adsorption of polycyclic aromatic hydrocarbons from wastewater by using silica-based organic–inorganic nanohybrid material. J Water Reuse Desalin. 2015;5:50–63.
45. Cheng G, Yang C, Wang X, et al. One-step synthesis of functional metal organic framework composite for the highly efficient adsorption of tylosin from water. J Colloid Interface Sci. 2021;586:269–278.
46. Neyens E, Baeyens J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater. 2003;98:33–50.
47. Ji F, Li C, Zhang J, Deng L. Heterogeneous photo-Fenton decolorization of methylene blue over LiFe(WO4)2 catalyst. J Hazard Mater. 2011;186:1979–84.
Fig. 3The interactive effect of (a) NH2-MIL-88B(Fe) amount (2.0 μL/mL of H2O2, 60 mg/L of IMC concentration, pH 6.4); (b) IMC concentration (0.3 g/L of NH2-MIL-88B(Fe), 2.0 μL/mL of H2O2, pH 6.4); (c) H2O2 amount (0.3 g/L of NH2-MIL-88B(Fe), 60 mg/L of IMC concentration, pH 6.4); (d) pH (0.3 g/L of NH2-MIL-88B(Fe), 60 mg/L of IMC concentration, 2.0 μL/mL of H2O2) on the removal percentage of IMC. 
Fig. 4The interactive effect of (a) NH2-MIL-101(Fe) amount (2.0 μL/mL of H2O2, 60 mg/L of IMC concentration, pH 6.4); (b) IMC concentration (0.24 g/L of NH2-MIL-101(Fe), 2.0 μL/mL of H2O2, pH 6.4); (c) H2O2 amount (0.24 g/L of NH2-MIL-101(Fe), 60 mg/L of IMC concentration, pH 6.4); (d) pH (0.24 g/L of NH2-MIL-101(Fe), 60 mg/L of IMC concentration, 2.0 μL/mL of H2O2) on the removal percentage of IMC. 
Fig. 5Influence of radical scavengers on IMC degradation with (a) NH2-MIL-88B(Fe) and (b) NH2-MIL-101(Fe). 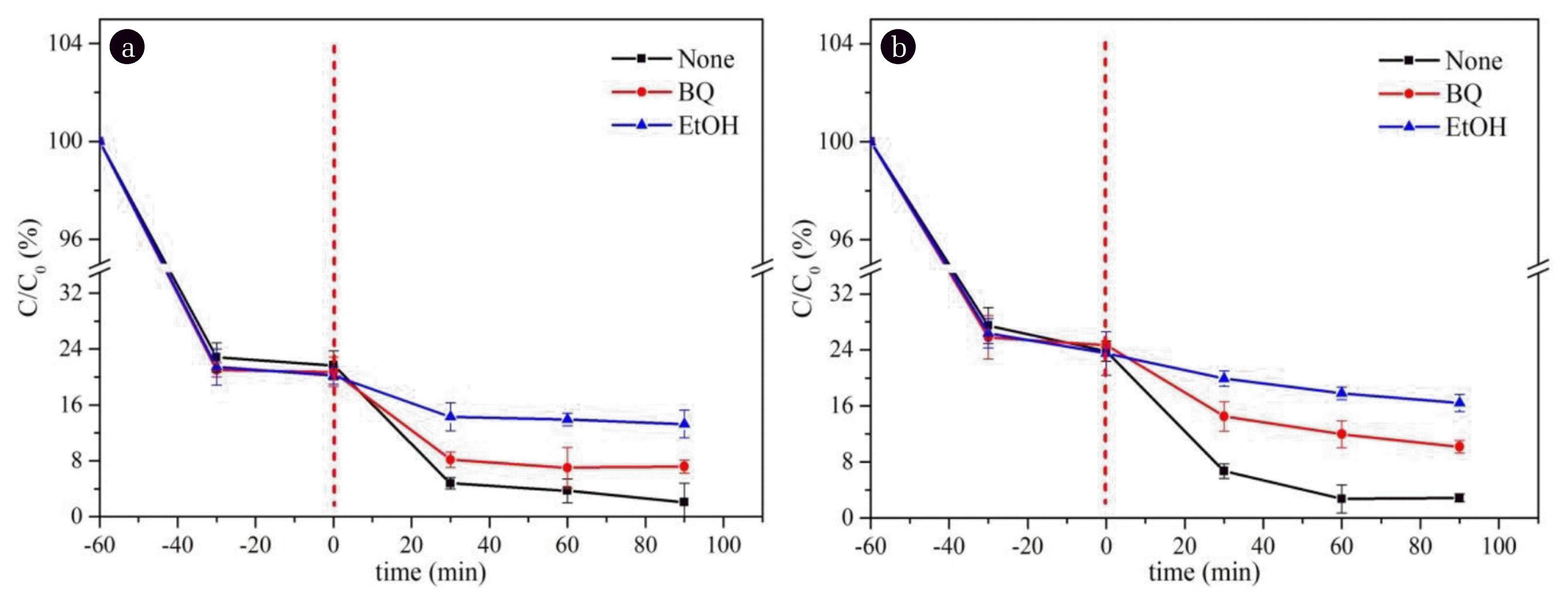
|
|
||||||||||||||||||||||||||||||||||||||||