1. Introduction
Hydrogen sulfide (H2S) in gases is a harmful and malodorous substance with a variety of sources such as waste landfills, wastewater treatment facilities, anaerobic digestion facilities, and oil and gas production processes [1–6]. In particular, biogases, including landfill gas and anaerobic digestion gas, contain H2S at a high concentration of hundreds to tens of thousands of ppm [2, 7–11]. Before biogas is used as a fuel, its H2S concentration must be reduced to an acceptable level. For example, the desirable H2S concentration of biogas is < 1,000 ppm for direct combustion by a boiler, < 100 ppm for an internal combustion engine, < 16 ppm for transportation fuel [7, 9, 11], and < 0.5 ppm for fuel cell [9, 10, 12, 13]. On the other hand, H2S concentration of the syngas produced by the pyrolysis and gasification of biomass or waste is often hundreds ppm and also needs to be removed. Otherwise, it will cause problems with the expensive catalysts, which is used for upgrading and the methanation of syngas [14, 15].
Adsorption by various sorbents such as zeolite, metal oxides, and activated carbon (AC) is mainly used in dry desulfurization to remove H2S from gases without the generation of any liquid waste [10, 14, 16, 17]. AC has a large surface area (200–2,000 m2/g) and pore volume (0.1–1 cm3/g), thermal stability, and high H2S removal capacity, making it the most widely applied H2S adsorbent [2, 6, 8, 18, 19]. It can also impregnate alkaline substances, thereby increasing the H2S removal capacity by chemical adsorption or catalytic reaction [2, 3, 6, 9, 10, 13–15, 19, 20]. However, AC preparation requires additional heat supply depending on the high temperature for the activation (800–1,100°C for physical activation or 400–1,000°C for chemical activation) [21, 22], which raises the energy use and cost and impacts the environment [5, 8].
Interest has grown in using biochar derived from discarded biomass as an efficient adsorbent for removing H2S from gases because biochar is carbonized at relatively low temperature (under 700°C) without additional activation process, thereby lowering the cost [8, 18, 23, 24]. Several studies have reported the H2S removal by biochars produced from various biomasses, including coconut, almond, palm kernel, pistachio nutshell, wood, camphor, bamboo, rice hull [25, 26], leaf waste [8], food waste, sewage sludge, and livestock manure [6, 17, 27, 28]. However, since the structure of biochars is usually not as porous as that of AC, modification of them is needed to remove H2S more efficiently.
In previous researches, biochars have been modified by chemical, physical, or magnetic methods or impregnation with minerals to remove contaminants (e.g., heavy metals, phosphates, etc.). The modification of impregnation with minerals loads biochar with different minerals such as zero-valent metal and metal oxide using metal compound solution before or after the pyrolysis of the feedstock [29–33]. In the chemical methods, alkalis are used for activating biochar, not attaching basic substances to biochar [34, 35]. Although many studies have been conducted on the loading AC with alkalis by impregnation and reported enhancement of H2S removal from gases, any researches could not be found on biochar modification by alkali-impregnation. Therefore, it is necessary to confirm whether the adding an alkali to biochar is effective to increase the H2S removal capacity of biochar.
This study aimed to investigate the improvement of the H2S removal characteristics of modified rice hull biochar by impregnating alkalis of sodium hydroxide, potassium hydroxide, sodium carbonate, or potassium carbonate. The H2S removal capacities of the alkaline impregnated biochars (IBCs) were determined by adsorption tests and compared with those of commercial coconut shell AC, which is used for biogas desulfurization. We also estimated the effects of the height-to-diameter (H/D) ratio of the packed adsorbent, inlet gas flow rate (i.e., linear velocity), H2S concentration of inlet gas, temperature, and inlet gas moisture (i.e., relative humidity (RH)).
2. Materials and Methods
2.1. Original Biochar and Activated Carbon
Biochar (BC) was a commercial product (Yuki Industrial Co., Ltd., Korea) that is mainly used for soil improvement and which is manufactured by carbonizing rice hull, a byproduct of rice polishing, under temperature conditions of 400 to 500°C. The BC particle size was measured to be less than 1 mm after screening, but often maintained the elongated shape similar to raw rice hull. AC was a commercial adsorbent (Dongyang Carbon Co., Ltd., Korea) made by coconut shell and crushed into 4×8 mesh particle size to remove H2S from biogas.
Bulk density was determined by repeating measuring the weight of material filled in a 500 mL-measuring cylinder three times. The bulk densities of the BC and AC were measured at 0.1144 kg/L and 0.4707 kg/L, respectively. The weight per unit volume of the BC was only a quarter of that of the AC. The volatile solid contents of the BC and AC were 60.7% and 94.0%, and their ash contents were 39.3% and 2.7%, respectively. The rice hull biochar would be less efficient in the production of activated carbon due to its relatively low volatile solid content and high ash content [17]. The ultimate analysis results revealed a carbon content of 55.61% for the BC but 84.51% for the AC, which was a significant difference. Meanwhile, metal oxides capable of reacting with H2S were not detected in the BC or AC as a results of X-ray diffraction spectroscopy (XRD) analysis.
2.2. Preparation of Alkaline Impregnated Adsorbents
The IBCs were prepared by adding NaOH, KOH, Na2CO3, or K2CO3 as alkaline impregnants to the BC using the incipient wet impregnation process [36, 37]. These four alkalis were found to effectively remove H2S from gases when adhered to AC [2, 19, 20, 36, 38–41]. Before the impregnation, the biochar was washed several times with distilled water to remove its impurities, dewatered and dried at 105°C for 24 h, and cooled at room temperature. Then, 15 g of the dried biochar was added to 500 mL of each alkaline aqueous solution in the following predetermined concentration: 1, 2, 3, 4, 5, 6, 7, and 10 M for sodium hydroxide, 1, 2, 5, and 10 M for potassium hydroxide, 1 and 2 M for sodium carbonate, and 1, 2, and 5 M for potassium carbonate within the range of solubility of the alkalis. In a 1 L beaker, it was stirred for 6 hours at room temperature (around 30°C) and 150 rpm to allow sufficient contact between the alkali in the solution and the biochar. After stirring, the mixtures were filtered to separate the biochar impregnated with alkaline substances, and the IBC samples were prepared by drying at 105°C for 24 h and cooling to room temperature in a desiccator [2, 36]. Impregnated AC (IAC) was made by attaching NaOH to the AC using the same impregnating method with 5 M NaOH solution to compare with the IBCs. The IAC was marked IAC/5M-NaOH.
2.3. Adsorption Test for H2S Removal
Continuous flow adsorption column tests were performed to evaluate the H2S removal characteristics, i.e. breakthrough time and adsorption capacity for the BC, IBCs, AC, and IAC. The length of the column was 160 mm, with an inner diameter of 16.57 mm made of acid-resistant stainless steel. Test columns were packed with each adsorbent at a packing H/D ratio of 2.0. For fixing the adsorbent in the column, both ends were filled with quartz wool. The packed column was connected to the gas lines, and the temperature kept 30°C in the chamber. The mixed gas was prepared by diluting 1,000 ppm of H2S to high-purity nitrogen and injected continuously into the column bottom at 600 mL/min using a gas flow meter for necessary adjustment. The effluent gas passing through the column was sampled periodically with a gas-tight syringe, and the H2S concentration was analyzed. In order to ensure the reliability of the test, three replicates for the H2S adsorption experiment were conducted under the same conditions, and the results were shown as the mean and standard deviation.
2.4. Determination of H2S Removal Characteristics
The H2S removal capacity means the amount of H2S adsorbed during the time until the H2S concentration of the effluent gas from the adsorbent reaches the inlet level. It represents the maximum amount of H2S removal per unit weight of the adsorbent and can be obtained using the breakthrough curve from the adsorption test and the following Eq. (1) [26, 37].
where q is the H2S removal capacity of adsorbent (mg/g); Q, the gas flow rate (mL/min); w, the amount of adsorbent (g); MW, the mole mass of H2S (34.08 mg/mmol); VM, the molar volume of gas (22.4 mL/mmol at 0°C, 1 atm); C0, the inlet level of H2S (ppm); C(t), the outlet level of H2S (ppm); and ts is the adsorption saturation time (min).
The adsorbent in the actual adsorption process is not replaced when the H2S removal capacity is entirely exhausted, but at the breakthrough time, which is when the desired breakthrough concentration in the effluent gas is reached. In this study, the breakthrough time was estimated as the time when the effluent H2S concentration got to 5% of the inlet level.
2.5. Analysis Methods
The amount of alkali loaded on the rice hull biochar was determined by measuring the weight change before and after impregnation. The surface area, pore size, and pore volume of the adsorbents were estimated by Brunauer-Emmett-Teller (BET) analysis. Their components were analyzed by X-ray fluorescence (XRF).
For the H2S analysis, a gas chromatograph (GC-17A, SHIMADZU, Japan) equipped with an Rtx-1 Capillary GC Column (30 m × 0.32 mm ID × 5 μm df) and a flame photometric detector (FPD) was used. The carrier gas was analytical high-purity helium. The injector temperature was 200°C. The column oven and detector were operated at 50°C (isothermal) and 220°C, respectively. Standard gases for H2S quantification were prepared in various concentrations with high-purity nitrogen as a dilution gas.
3. Results and Discussion
3.1. Properties of Adsorbents
We examined the material properties and composition of the BC, IBCs, AC, and IAC. The un-impregnated BC and AC were basic [25, 26], and the pH increased further after alkali was impregnated onto the BC and AC. Therefore, all these adsorbents have alkaline properties suitable for enhancing the probability of dissociation and adsorption of gaseous H2S [6, 20, 25, 42].
Before H2S adsorption, no sodium was detected in the BC. Meanwhile, the sodium content was 23.4% in IBC/5M-NaOH, which was much higher than 6.2% in IAC/5M-NaOH. No sulfur was found in the adsorbents before the test.
The BC had a surface area of 83 m2/g, similar to that of rice hull biochar used in another study [26]. Because it was made only by carbonization without activation, its surface area was very small compared to that of the AC, which gave it a relatively low H2S removal capacity [25]. However, following the impregnation of NaOH, the surface area of IAC/5M-NaOH was reduced by 27% (828 m2/g), while that of IBC/5M-NaOH was increased by more than 12 times to 1,034 m2/g, slightly less than that of the AC (1,123 m2/g). Impregnating alkaline agents onto AC decreases the surface area [20, 39, 41], because the impregnants adhere to the surface and micro-pores of AC and block the inlet of meso-pores and micro- pores [20, 37]. Impregnated alkalis increase the chemical adsorption despite reducing the surface area of AC, which improves the overall H2S removal capacity [41, 43]. In contrast, the IBC was afforded a more favorable condition for H2S removal due to the increased surface area by the alkali impregnation.
The average pore size of the IAC increased from 1.69 nm to 1.98 nm before and after the impregnation of alkali. Still, the total pore volume decreased because the NaOH crystals that formed on the surface of AC during the impregnation blocked the micro-pores [2, 15, 40, 44]. Unlike the IAC, the average pore size and total pore volume of the IBC/5M-NaOH increased from 2.33 nm to 35.90 nm and from 0.05 cm3/g to 2.40 cm3/g more than before the impregnation, respectively. As NaOH adheres to the surface of biochar, relatively large pores are developed, which increases the effective diffusivity of H2S and delays the pore blockage due to adsorption, thus maintaining a higher removal capacity [17, 43]. The pore volume of IBC/5M-NaOH (2.40 cm3/g) was greater than those of IAC/5M-NaOH (0.42 cm3/g) and AC (0.47 cm3/g), thereby providing a large reaction area for chemical adsorption of H2S [14].
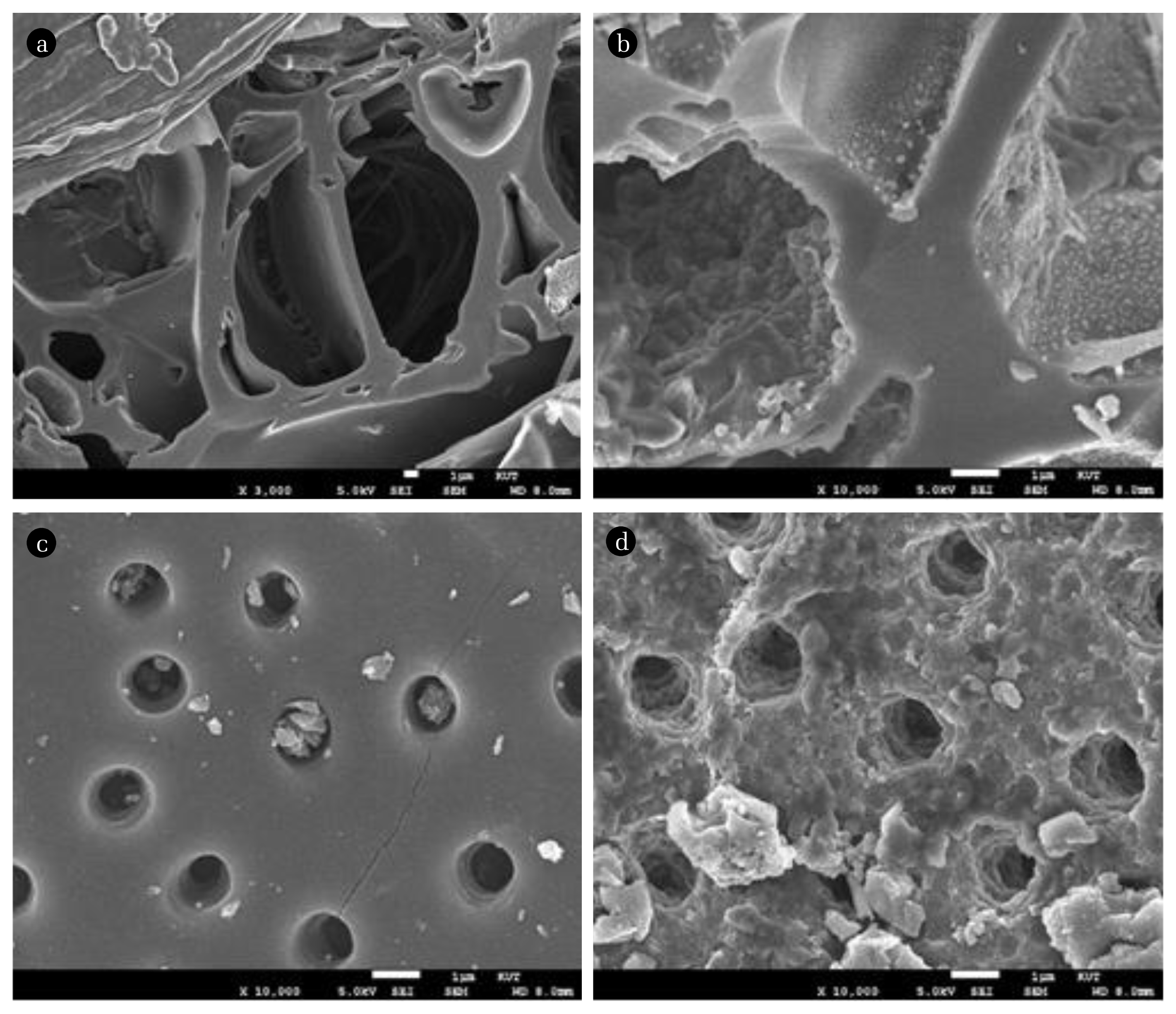
Surface changes resulting from NaOH deposition were also observed in the scanning electron microscope (SEM) image (see Fig. 1). The BC maintained the structure of the rice hull itself with its very poor micro-pores, because it was produced with only carbonization process. On the other hand, the AC manufactured with activation had well-formed and evenly distributed micro-pores, so it showed a more favorable porosity for physical adsorption of H2S than the BC. After impregnation with NaOH, the shape of the IBC did not differ much from that of the BC. However, the formation of NaOH crystals over the entire surface leaded to a large increase in the surface area of the IBC. The IAC also showed the shape of the AC as it is, but since the surface was covered with NaOH deposits, the micro-pores of the AC were narrowed or blocked.
3.2. H2S Removal Characteristics
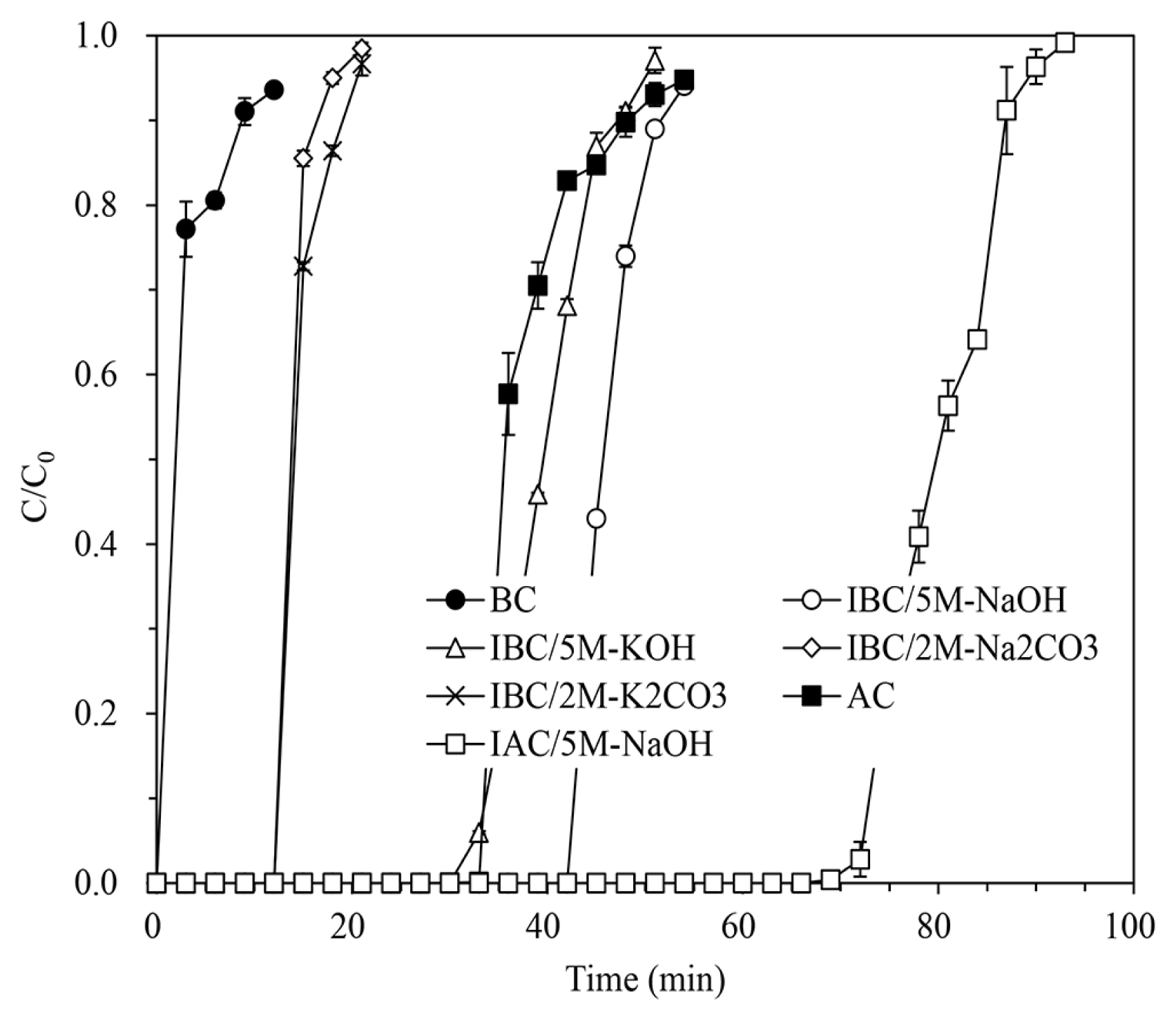
The H2S breakthrough curves of the column tests for some adsorbents such as BC, IBC/5M-NaOH, IBC/5M-KOH, IBC/2M-Na2CO3, IBC/2M-K2CO3, AC, and IAC/5M-NaOH are shown in Fig. 2 in the dimensionless concentration (C/C0), which is the ratio of outlet to inlet concentration over time. All the adsorption tests showed the dependence of the H2S removal capacities on the adsorbent and the impregnated conditions (see Table S1). The un-impregnated BC exhibited a very small H2S removal capacity of 2.73 ± 0.10 mg/g, which was only about 36% of the original AC’s capacity of 7.65 ± 0.16 mg/g. The BC was produced only by carbonization, so the formation of micro-pores was relatively poor, and its physical adsorption of H2S in the gas was far less than that of the AC. The removal capacity of IAC/5M-NaOH was 14.38 ± 0.09 mg/g due to the improvement of chemical adsorption capacity by the NaOH impregnation onto the AC [41, 45].
All alkalis showed a much higher impregnation effect on the BC, as the case of the AC, for the H2S removal capacities. Although the H2S removal capacities varied depending on the kind of alkali and concentrations of the alkaline agent solution, all of the IBCs prepared with the lowest alkali solution concentration, i.e. IBC/1M-NaOH, IBC/1M-KOH, IBC/1M-Na2CO3, and IBC/1M-K2CO3 had a three-fold greater H2S removal capacity compared to the BC. Among the IBCs, IBC/5M-NaOH exhibited the highest H2S removal capacity of 24.4 ± 0.05 mg/g, which is about 3.2 times that of the AC and even about 70% greater than IAC/5M-NaOH prepared with the same concentration of the alkali solution. This result confirmed that the alkali impregnation onto rice hull biochar, without requiring any activation process, greatly enhances H2S adsorption capacity of the biochar.
The breakthrough time at maximum H2S removal capacity by the adsorbents decreased in the order of IAC/5M-NaOH (72.28 min) > IBC/5M-NaOH (42.35 min) > AC (33.58 min) > IBC/5M-KOH (32.53 min) > IBC/2M-K2CO3 (12.21 min) > IBC/2M-Na2CO3 (12.18 min) > BC (0.19 min). Although the removal capacity of IBC/5M-NaOH was greater than that of IAC/5M-NaOH, the breakthrough time of IAC/5M-NaOH was greater than that of IBC/5M-NaOH. It is because the bulk density of IAC/5M-NaOH (0.5192 kg/L) was much greater than that of IBC/5M-NaOH (0.1767 kg/L). Nevertheless, the breakthrough time of IBC/5M-NaOH was larger than that of the AC with greater bulk density, which demonstrated the excellent H2S removal property of alkaline IBC.
After the adsorption, the sulfur content was 0.2% for the AC, 1.9% for IAC/5M-NaOH, and 6.5% for IBC/5M-NaOH. This sulfur was caused by the immobilization and accumulation resulting from H2S adsorption by chemical reaction with the alkali on the adsorbents surface [27].
When the alkali on rice hull biochar is sodium hydroxide or potassium hydroxide, H2S in gas can be chemically adsorbed by one of the following two reactions [40].
or
If sodium carbonate or potassium carbonate is impregnated on biochar, the reaction between H2S in gas and the alkali can be as follows.
or
where A is sodium or potassium.
Tsai et al. [40] presented (Eq. (2)) as the primary reaction since 1 mol of H2S removed corresponds to 1 mol of NaOH or KOH by the H2S adsorption test for impregnated activated carbon. The sodium hydroxide or potassium hydroxide loaded on rice hull biochar will also react with H2S according to Eq. (2). When Na2CO3 or K2CO3 is impregnated on rice hull biochar, 1 mol of H2S and 1 mol of alkali correspond in both Eq. (4) and (5). As a result, 1 mol of each alkali on the IBC in this work reacts with 1 mol of H2S.
Using the H2S removal quantity of each IBC and the reaction stoichiometry, we evaluated the proportion of the alkali consumed to remove H2S out of the total amount of impregnated alkali. The fractions in IBC/5M-NaOH, IBC/5M-KOH, IBC/2M-Na2CO3, and IBC/2M-K2CO3 were estimated as 6.12% of NaOH, 7.86% of KOH, 22.15% of Na2CO3, and 39.52% of K2CO3, respectively. Contrary to the studies of IAC [36, 40], only some of the added alkali of IBCs was used for H2S adsorption.
3.3. Effect of Impregnated Alkali
In order to investigate further the variation in H2S removal according to the alkalis used, we examined the adsorption test results for the IBCs prepared with the same 2 N of alkaline solution. Considering the mole ratio of sodium and potassium reacting with H2S, the H2S removal characteristics of IBC/2M-NaOH, IBC/2M-KOH, IBC/1M-Na2CO3, and IBC/1M-K2CO3 were compared. The removal capacity of H2S was 13.50 ± 0.03 mg/g for IBC/2M-NaOH, which was larger than 10.03 ± 0.05 mg/g for IBC/1M-Na2CO3, 9.41 ± 0.01 mg/g for IBC/2M-KOH, and 8.81 ± 0.02 mg/g for IBC/1M-K2CO3. The breakthrough times decreased from a maximum of 15.18 min for IBC/2M-NaOH to 12.13 min for IBC/2M-KOH, 9.18 min for IBC/1M-K2CO3, and 9.14 min for IBC/1M-Na2CO3.
When NaOH was impregnated onto rice hull biochar, it showed the best H2S removal characteristics among the selected alkalis. The sodium hydroxide attached to rice hull biochar was 6.00 meq/g, which was more than twice the 2.85 meq/g of potassium hydroxide with the second largest amount of alkali and much more than 0.75 meq/g of sodium carbonate and 1.06 meq/g of potassium carbonate. In a previous study on impregnated activated carbon, NaOH-IAC was reported to have a larger H2S removal capacity than the adsorbents with KOH, Na2CO3, and K2CO3 because the molecular size of NaOH is relatively small compared to that of the others, which eases the transportation into the pores [38]. Even when using the alkali solution of the same concentration, the degree of adhesion to the rice hull biochar can vary depending on the alkali, and therefore can affect the H2S removal from gas.
In addition, Eq. (2) and (3) show that water is produced and is absorbed into the impregnated material to form an alkaline water film. H2S is transferred to the film from gas, dissolved and dissociated, and reacts with the alkali [2, 14, 17, 38, 42]. The solubility of sodium hydroxide, which is greater than the other alkalis used in this study, resulted in the best H2S removal performance.
3.4. Effect of Impregnated Alkali Amount
At the higher concentration of alkali solution, the amount of alkali attached to the rice hull biochar surface increased linearly, as also for activated carbon [37]. Especially, in the case of NaOH and KOH, the Pearson correlation coefficients between the concentration of impregnant solution and the loaded amount of alkali exceeded 0.98 (p < 0.01), indicating a high correlation.
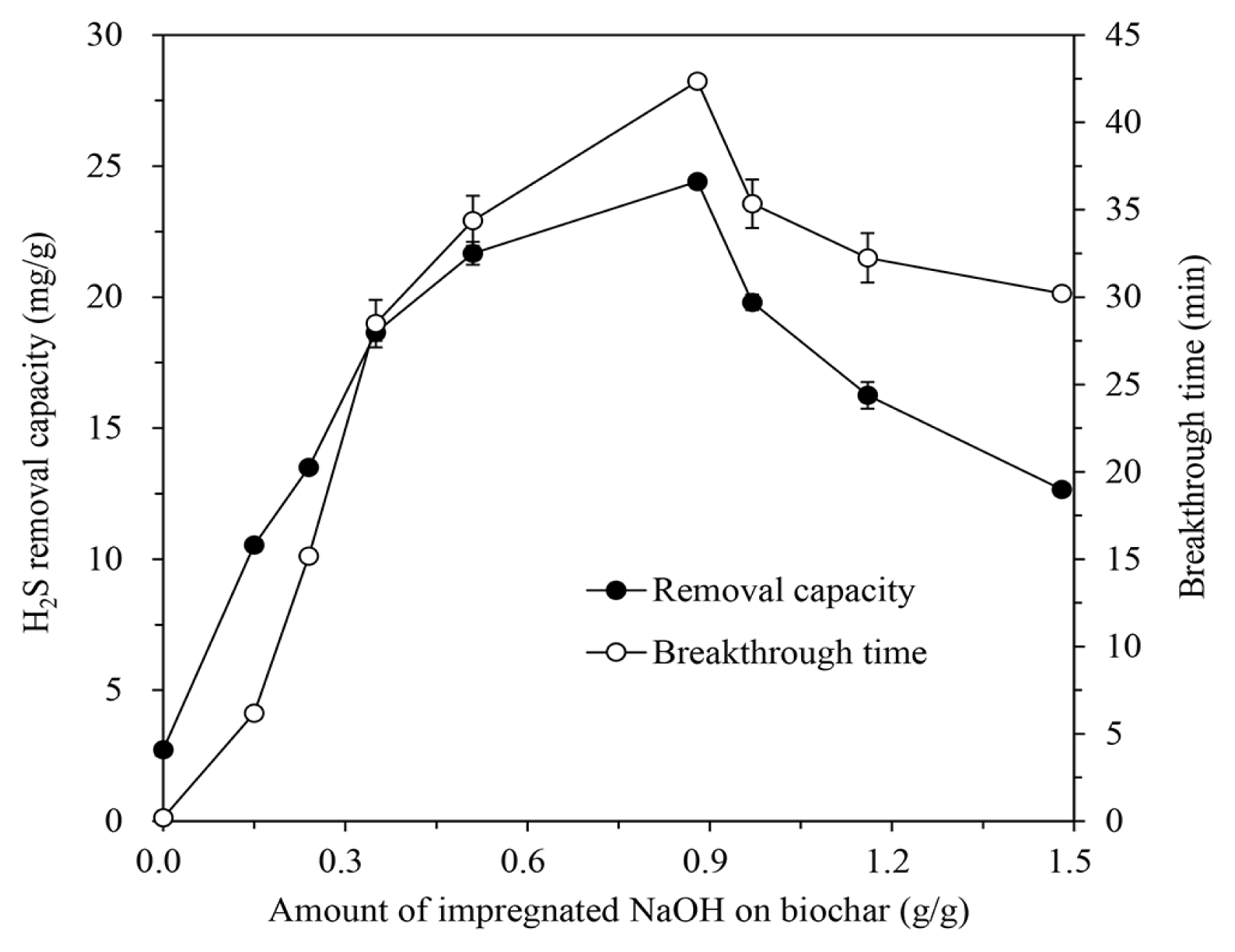
However, the increase of impregnated alkali amount did not directly improve the H2S removal characteristics of the IBCs. For the NaOH-IBC which showed the best H2S adsorption performance, the H2S removal capacity and breakthrough time increased up to a NaOH solution concentration of 5 M, but then decreased with further solution concentration increase, as shown in Fig. 3.
The amount of added alkali on rice hull increases with increasing the alkali concentration of solution for impregnation, which leads to extent the effective surface area that can adsorb H2S. This results in the augment of H2S removal capacity and breakthrough time. As the amount of alkali increases, the loaded alkali saturates or blocks the pores of biochar. Since the pores of the biochar decrease and the additional alkali builds up on the saturated surface, the number of available active sites decreases, like impregnated activated carbon [2, 20]. Therefore, H2S removal capacity and breakthrough time of the IBC also decline again, because the H2S molecules in gas cannot penetrate into the pores and only react with the alkali on the surface rather than in the pores.
3.5. Effect of Adsorption Conditions
The effects of the adsorbent H/D ratio, inlet gas flow rate, H2S concentration of inlet gas, temperature, and inlet gas moisture on the H2S removal characteristics were estimated using IBC/5M-NaOH, which had the best H2S removal capacity in this study.
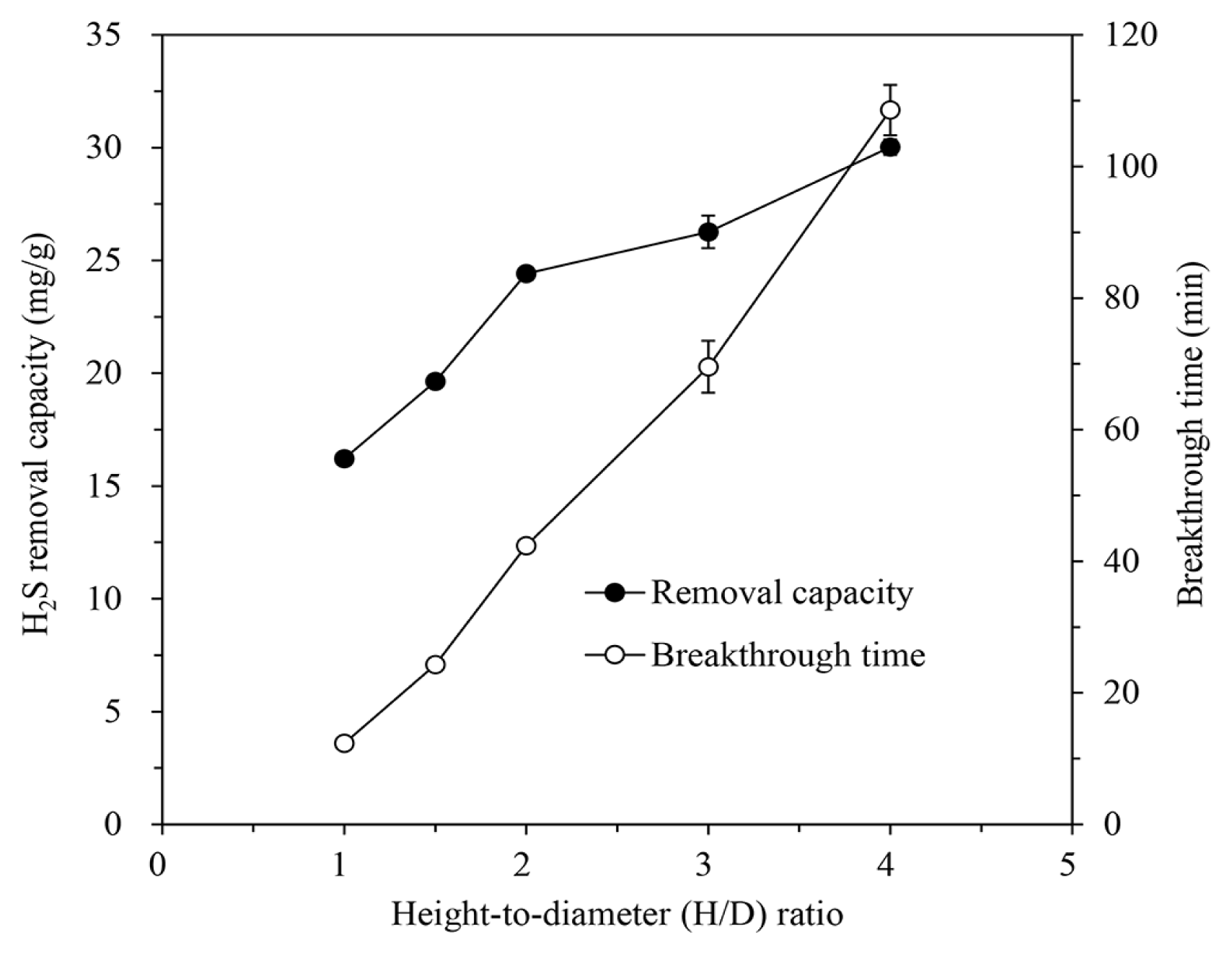
IBC/5M-NaOH was packed into the column with an H/D ratio of 1.0, 1.5, 2.0, 3.0, and 4.0, and the H2S removal characteristics were evaluated by introducing the gas containing 1,000 ppm of H2S at a flow rate of 600 mL/min at 30°C. As shown in Fig. 4, the removal capacity increased up to 30.02 ± 0.35 mg/g at an H/D ratio of 4.0, but was 16.22 ± 0.04 mg/g at an H/D ratio of 1.0. The breakthrough time lengthened linearly as the H/D ratio, i.e. the volume of the packed IBC increased. At an H/D ratio of 1.0, the breakthrough time was 12.32 min, but at 4.0, it increased more than eight times to 108.56 min because of the increasing number of active sites of IBC/5M-NaOH that can react with H2S in gas [19, 46]. Furthermore, as the amount of packed IBC/5M-NaOH increases, the contact probability between H2S and NaOH on the IBC increases, allowing sufficient time for reaction and even distribution of the gas within the adsorbent layer [2, 10].
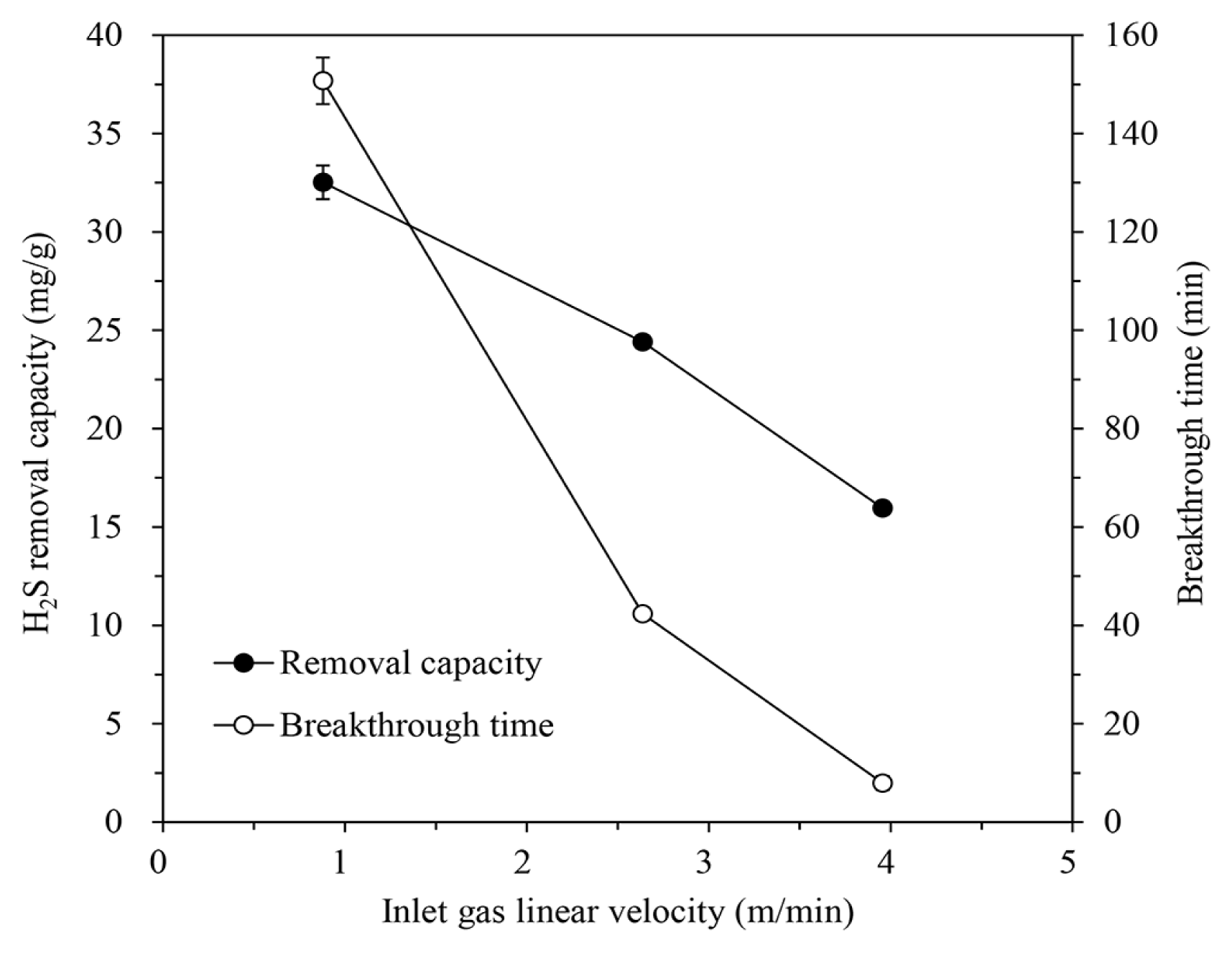
To examine the effect of the inlet gas flow rate, the gas was passed through the column packed with IBC/5M-NaOH (H/D ratio of 2.0) at a flow rate of 200, 600, or 900 mL/min, equating to a linear velocity of 0.88, 2.64, and 3.96 m/min, respectively. The H2S concentration of the inlet gas was 1,000 ppm, and the temperature was maintained at 30°C. The results of the adsorption tests were shown in Fig. 5. Both the H2S removal capacity and breakthrough time were decreased as the gas flow rate (linear velocity) increased. For the chemical reaction, a sufficient contact time is required between the H2S in gas and the NaOH on the IBC [2, 19, 37]. Even if the gas flow rate is large within a certain range, all H2S molecules can contact and react with NaOH at the beginning stage. As the active sites of the IBC is exhausted, however, the probability of contact between H2S and NaOH decreases rapidly. Therefore, if the gas linear velocity is too fast and the contact time is insufficient, all of H2S in gas cannot be removed and the H2S removal capacity is also reduced.
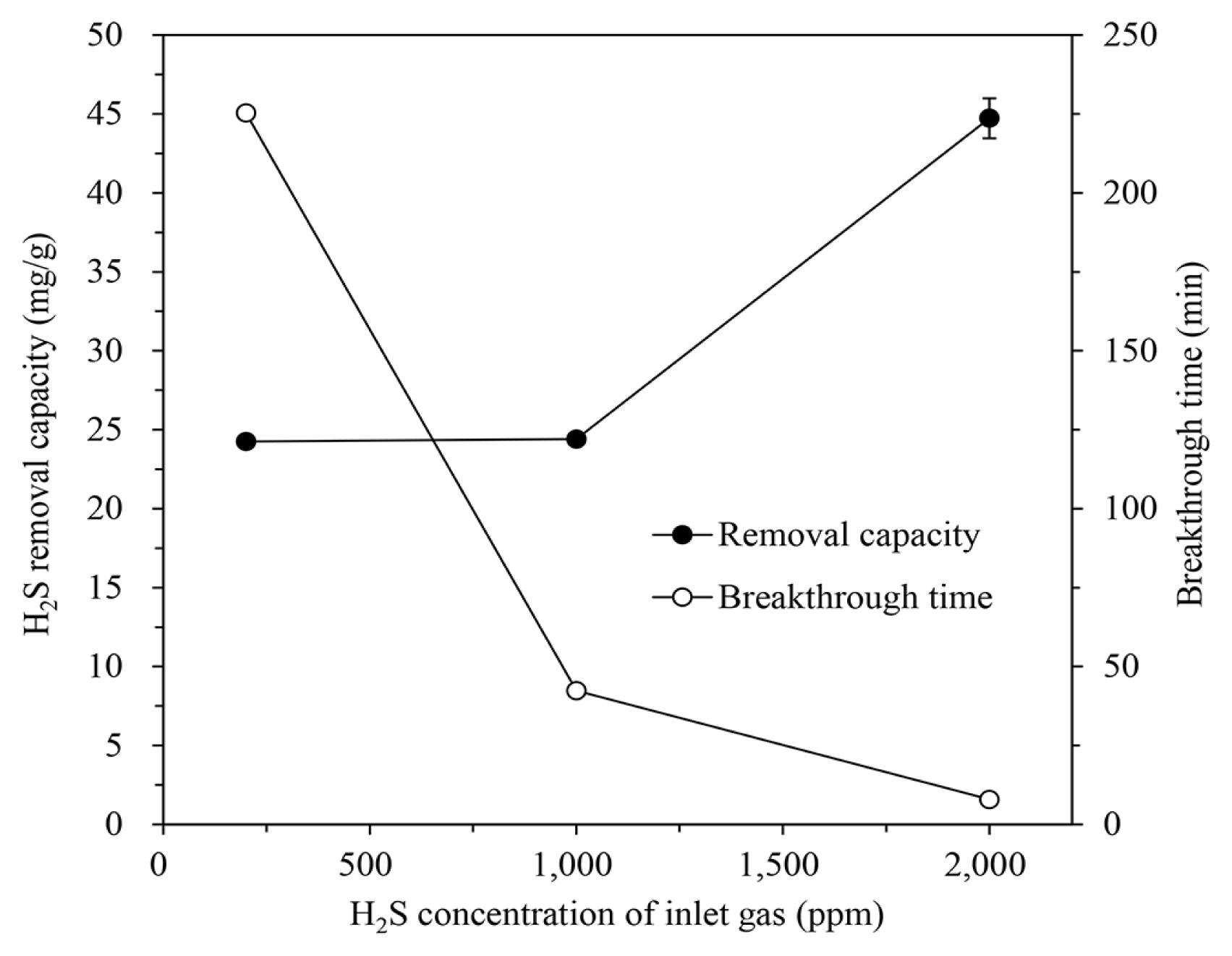
Adsorption tests were carried out using the gases containing H2S at 200, 1,000, and 2,000 ppm to investigate the effect of the H2S concentration of the inlet gas on the H2S removal of the IBC. These gases were introduced into the column packed with IBC/5M-NaOH at an H/D ratio of 2.0, at a flow rate of 600 mL/min and a temperature of 30°C. Increasing the H2S concentration of the inlet gas, as shown in Fig. 6, reduced the breakthrough time as the H2S removal capacity was quickly consumed. Even increasing the H2S concentration from 200 ppm to 1,000 ppm did not significantly affect the removal capacity, but a further increase to 2,000 ppm did sharply increase the capacity to 44.73±1.26 mg/g. The lower the H2S concentration in the inlet gas, the longer the adsorption duration until the removal capacity is exhausted. Rising the inlet H2S concentration increases the removal capacity due to the increased effective diffusivity of H2S and the mass-transfer flux of gas to the IBC surface [2, 36, 38].
The change of H2S removal characteristics of IBC/5M-NaOH under different temperature conditions is shown in Fig. 7. In the adsorption test, the temperature was adjusted to 10, 30, and 50°C while maintaining the H2S concentration of 1,000 ppm, gas flow rate of 600 mL/min, and H/D ratio of 2.0. As the temperature was increased, both the H2S removal capacity and breakthrough time increased. The IBC removes H2S from gas by chemical adsorption, so the higher the temperature, the more the reaction between H2S and NaOH is promoted, as well as alkaline impregnated activated carbon [2].
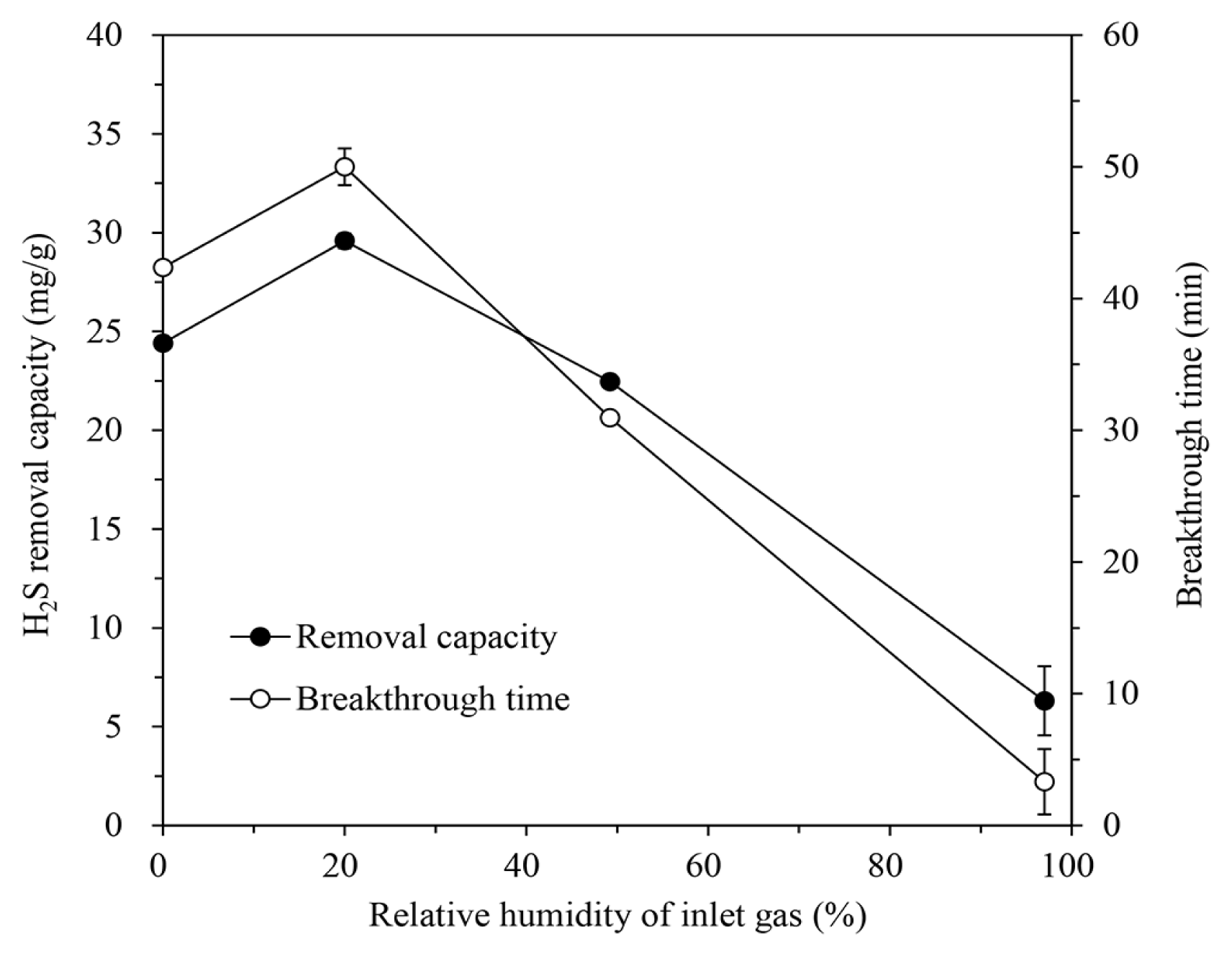
After increasing the RH by supplying moisture to the inlet gas, the H2S removal of the IBC/5M-NaOH was estimated. The adsorption tests were conducted at an H/D ratio of 2.0, inlet H2S concentration of 1,000 ppm, inlet gas flow rate of 600 mL/min, and temperature of 30°C. The removal capacity and breakthrough time increased at 20.0% RH compared to that of the dry conditions (see Fig. 8). On the contrary, the additional moisture increase degraded the H2S removal characteristics to a very low level at 97.1% RH. A certain level of moisture can serve to help the absorption and dissociation of H2S and the reaction with loaded NaOH [20, 27]. At excessive RH in gas, however, smaller molecular weight of H2O compared to that of H2S may enable it to diffuse faster than the adsorbate. As water occupies the pores and surfaces of the IBC, the reactive area for H2S adsorption may decrease [6, 10, 13].
4. Conclusions
We added four alkalis (sodium hydroxide, potassium hydroxide, sodium carbonate, or potassium carbonate) to rice hull biochar and examined the removal characteristics for H2S from gas. The H2S removal capacity of alkaline impregnated biochars far outperformed that of original biochar (2.73 mg/g) and of commercially available coconut shell activated carbon (7.65 mg/g). Sodium hydroxide had a greater degree of adhesion onto the rice hull biochar and a higher H2S removal capacity than those of the other three tested alkalis. The maximum removal capacity of the IBC prepared with 5 M of NaOH solution reached 24.41 mg/g. The removal characteristics of the NaOH-IBC were affected by the H/D ratio of the adsorbent, inlet gas flow rate, H2S concentration of inlet gas, temperature, and gas humidity.