1. Introduction
Ozone is a strong oxidizing agent, with a redox potential of 2.07 V compared to the normal hydrogen electrode (NHE) [1]. It is used in the wastewater treatment industry for degradation of dyes and toxic reagents [2, 3]. Conventional method for the ozone generation is cold corona discharge which has some limitations such as high initial cost, releasing ozone into the air, production of harmful nitrogen oxides, low ozone concentration, low process efficiency. Electrochemical ozone generation is a new approach for resolving the limitations of cold corona discharge process. Electrochemical ozone generation was performed in the electrochemical cell containing two electrodes (anode and cathode) in presence of appropriate electrolyte [4, 5]. During electrolysis, a variety of oxidants can be produced depending on the reaction condition. The generation of such oxidants strongly depends on the several key reaction parameters, including electrode material, electrolyte composition, applied current (or voltage), pH, temperature, and type of electrolysis. Among these parameters the electrode material has important effect on kind and yield of generated oxidant [6]. In electrochemical ozone generation, anions in electrolyte should not engage in competitive reactions with oxygen evolution or electrochemical ozone generation and cations with hydrogen evolution reaction [7]. For these reasons, we used HClO4 (0.1 M) as electrolyte that reported in other publications without interference for electrochemical ozone generation [8–10].
In addition, the most important part of the electrochemical ozone generation system is the anode electrode because ozone is generated from water splitting at the surface of the anode. The anodic material used in this system should have some properties such as high over potential for oxygen evolution, good conductivity, being inert and stable to anodic polarization in the electrolyte [1]. Up to now, several anode materials have been used, such as lead dioxide [11], platinum [12], boron-doped diamond [13], glassy carbon [14] and titanium types [4], but the highest efficiency belongs to the titanium electrode modified with nickel, antimony and tin oxides [1, 4, 15, 16].
In this research, a novel Ti/TiHx/SnO2-Sb2O5-NiO-CNT modified titanium electrode containing carbon nanotubes (CNT) was fabricated. In addition, with adding TiHx sublayer stability of the modified electrode increased. The modified electrode was used an anode for in-situ ozone generation in presence of HClO4 (0.1 M) as electrolyte. So, this generated ozone can degrade organic pollutant produced from textile process.
One of the most important environmental challenges in textile industries is the production of toxic wastewater because of using different dyes and chemicals in textile related processes [17, 18]. Textile dyes are used in the dyeing procedures have different classifications such as azo and anthraquinone. Azo dyes are known by N=N groups in their chemical structure and constitute 60–70% of the textile dyestuff produced [19–21]. An important group of azo dyes in textile industry is reactive dyes, which used for dyeing a wide range of natural and synthetic fibers such as cotton, wool, nylon etc. Dye fixation on the substrate in reactive dyeing procedures is not 100 percent and significant quantity of hydrolyzed reactive dyes will remain in the dyebath at the end of the dyeing procedure which needs to be washed off the textile materials and discharged into the effluent [22, 23].
Traditional wastewater treatment methods often were unable to remove synthetic dyes completely due to their complex chemical structure [24, 25]. Therefore, it was necessary to introduce some new methods that can degrade big molecules of toxic dyes and make them ready to be released to the environment safety. As known, microbial fuel cell (MFC) is an innovative environmental and energy system in which biodegradable organic compounds are converted into electrical energy via microbial catalysis. Some researchers reported this type of electrode for wastewater treatment and energy generation [26–31]. One of these toxic dyes which widely used in the textile industry is RR 195. Different techniques were implemented to remove this dye from wastewater such as photocatalytic degradation [32, 33], electrofenton process [34], electrocoagulation process [35], bio-sorbent material [36] and electrochemical method [37]. N=N group in molecular structure of this dye can be oxidized by ozone and other radical species produced in the electrochemical method. In recent years, the application of electrochemical technology has been given more attention because of many reasons such as simple operation, low initial cost, high efficiency, no sludge residual and no needed chemicals.
Researchers introduced different anodes for electrochemical degradation of textile dyes. Maljaei et al. reported that C.I. reactive yellow 3 can be degraded by graphite electrode [38]. Rivera et al. studied the degradation of C.I. reactive black 5 and C.I. basic yellow 28 using diamond and metal alloy electrodes and reported successful degradation and mineralization of these dyes [39]. Rangel et al. [40] introduced boron-doped diamond (BDD) for electrochemical oxidation of C.I. reactive blue 222. Shetti et al. [41] studied on the electrochemical detection and degradation of C.I. direct red 28 by modified glassy carbon.
An important category of electrodes used for removal toxic compounds is Dimensionally Stable Anodes (DSA) [42]. They contain platinum and modified Titanium (Ti) coated with ceramic metal oxides film and nanomaterials. These group of electrodes were used for removal toxic compounds and metal ions such as; copper [43], ammonium nitrogen [44] and textile dyes [45–54]. The metal oxide-film electrodes such as tin oxide has good electrocatalytic properties related to chemical composition, the electronic structure and crystallinity of the oxide layer. Tin oxide and tin oxide doped with other materials have been well known for good electrical conductivity, low cost, high oxygen evolution potential, good electro-catalytic activity and ability in electro-generation of active oxygen. In addition, it can be prepared easily form hydrothermal pyrolysis in air atmosphere [1]. Several Ti electrodes modified with metal oxides base on tin oxide and nanomaterials were used for degradation of textile dyes such as; modification of Ti; with tin oxide for degradation of acid red 33 [45]; tin and iridium oxides for degradation of acid red 29 [46]; tin, ruthenium and iridium oxides for degradation of reactive orange 5, reactive red 3 BS, direct red 4BS, direct green BE, direct black 19, reactive blue 19 and disperse red 3 B [47]; tin, antimony and iridium oxides for degradation of acid blue 74 [7]; tin and tantalum oxides for degradation of of basic violet 10 [48]; tin and lead oxides for degradation of acid orange 52 [49]; tin and antimony oxides for degradation of reactive orange 7 [50]; tin, antimony oxides and CNT for degradation of acid red 73 [51]; tin, antimony and lead oxides for degradation of reactive red 195 [37]; tin, antimony, lead, bismuth oxides and CNT for degradation of acid orange 52 [52]; tin, antimony and nickle oxides for degradation of basic violet 10, reactive blue 50 and reactive red 198 [8, 53, 54] (for more detail please see Table S1).
Recently, the modified Ti electrode with tin, antimony, and nickel oxides is well known as a selective electrode for ozone generation and dye degradation [8, 53, 54]. Disadvantages of these group of electrodes are short service life time and low efficiency which limited their applications [55, 56]. A simple and effective process to improve the stability of these electrodes is to modify the substrate using different surface modifiers such as fluoridedoped tin oxide [57], TiO2 NTs [58, 59], TiHx [60, 61] etc. These modifiers will enhance the catalyst adhesion on to the substrate and improve the stability of the electrodes.
Also, due to specific properties of carbon nanotubes (CNT) such as high functional groups in structure, large surface area, electrochemical activity, high stability, high adsorption capacities and high electrical conductivity can lead to the improvement of life time and efficiency of the modified electrode [62, 63]. The researchers used (CNTs) for modifed Ti electrode for using in dye degradation [64, 65].
In this study we focused on electrochemical ozone production on Ti/TiHx/Sb-SnO2 electrode as a stable electrode. Presence of sublayer of TiHx improves the stability of electrode. With adding Ni and CNT to Mixed Metal Oxides (MMO), the surface area and therefore its active sites were increased for ozone generation. In addition, increasing CNTs to the metal oxides catalysts improves adhesion of catalyst layer to sublayer because of having high functional groups. At the first time, a stable modified Ti electrode (Ti/TiHx/SnO2-Sb2O5-NiO-CNT) was introduced for electrochemical ozone generation with high efficiency. In addition, generated ozone can break the double bond of azo chromophores and degrade RR 195 as a member of a large variety of toxic environmental pollutants.
2. Material and Methods
2.1. Chemicals
The titanium foil (Ti) with a thickness of 0.2 mm and purity of 98% was purchased from Boaji company china and other reagents included C2H2O4, C3H6O, NaOH, C4H10O, HNO3, Ti(OCH2 CH2CH2CH3)4, SnCl4, 5 H2O, NiCl, 6 H2O, SbCl3, CNT, C2H5OH, HCl, HClO4, K2Cr2O7, H2SO4, Ag2SO4, C16H7K3N2O11S3, NaH2PO4, H3PO4 were all analytical grade chemicals purchased from Sigma Aldrich and Merck companies and used without any purification. Textile dye with the specifications shows in Table S2 purchased and used without any purification. Also, deionized water was used to prepare all experimental solutions.
2.2. Apparatus
A wire-cut machine (WMT Metal, WMT CNC DK-7740, China) was used for cutting Ti samples. An ultrasonic batch (Ultrasonic cleaner, Ultra, China) was used for cleaning Ti electrode surface and preparation of the experimental solutions. Spin-coating machine (Backer, Vcoat 3, Iran) was employed for creating a thin and uniform layer on the Ti substrate. Drying and pyrolysis of the coated layers were executed in an oven (Paat Ariya Sanat, OV 55, Iran) and a furnace (Exciton, EX-1200-2L, Iran), respectively. Electrochemical ozone generation and dye degradation were performed with a coulometer (SAMA 500, Iran) performed in a UV Cell volume 4 mL. The UV-Vis spectrophotometer (Analytic Jena, Spcord 250, Germany) and a quartz cell with 1.0 cm length was used for recording UV-Vis spectrum of generated ozone. In addition, this instrument and quartz cell were used for measuring absorbance of dye solution and studying on dye degradation. A Potentiostate/Galvanostate (AUTOLAB, PGSTAT 302 N with NOVA 2.1 software, Netherland) was also used for electrochemical characterization of electrode. Surface morphology of the anode electrode was analyzed by scanning electron microscopy (SEM) (PHENOM, PRO X, Switzerland). Crystal structures prepared on the electrodes were studied by the X-Ray diffraction (XRD) method using CuK-α lamp under applied potential of 40 kV and current of 40 mA in 2θ range set in 10–90 degrees (Panalytical, X’PERT PROR MPD with software High Score Plus 3.0.5, Netherland).
2.3. Preparation of Ti/TiHx/SnO2-Sb2O5-NiO-CNT Electrode
The Ti plates first cut to dimensions of 8 × 8 mm, then washed with a mixture of acetone and sodium hydroxide (1M) with a volume ratio of 1: 1, for 10 min in an ultrasonic bath according to Li et al. [60]. Ti substrate then inserted into the boiling oxalic acid 10% (w/w) for 2 h and finally, the electrode was washed for 60 min with deionized water in an ultrasonic bath. Modification of the electrode was performed according to the method reported by Shao et al. [61]. To create TiHx sublayer on Ti substrate before coating with electrocatalyst modifier, the surface of the electrode was covered with a solution containing titanium (IV) butoxide 80% vol, 2-butanol 20% (v/v) and HNO3 (0.1M) in two steps spin-coating procedure (500 rpm for 20 s, then 1,500 rpm for 30 s). The electrode was then dried at 110°C for 10 min for solvent evaporation and to form TiO2 on the substrate. The pyrolysis process was performed at 500°C for 20 min in the furnace. Finally, the TiO2 layer was reduced electrochemically (two-electrode system; platinum was used as the counter electrode) in a 5% (w/w) sodium sulfate solution by applying cathodic current 10 mA for 5 min which leads to the formation of the TiHx layer on the substrate. In addition, a solution of different metal salts was prepared in absolute ethanol according to the Wang’s optimized values [4]. To prepare metal salt solution, 3.55 g of SnCl4·5H2O, 0.036 g of SbCl3 and 0.0048 g of NiCl2·6H2O were suspensioned in ethanol and then a few droplets of concentrated HCl were added to make the solution clear and absolute ethanol was then added to 10 mL in a volumetric flask. This mixture consisted of Sn:Sb:Ni in the molar ratio of 500:8:1. To optimize CNT, six volumetric containers were taken, 1 mL of the prepared metal salt solution was added to each container, and appropriate amounts of CNT were added to them that the molar ratios of CNT to Ni obtained to be 0, 5, 10, 15, 20 and 25. The solutions were sonicated for two h. Each of the new solutions was immediately used after sonication to modify the electrode by the two-step spin-coating process. After spin-coating, the electrode was dried at 110°C for 10 min and calcined at 500°C for 20 min. This procedure was repeated more than 22 times to ensure the full coverage of the geometric surface area of the electrode with the composite mixture. The number of repetitions was based on the thickness of the coated layer and the weight of the deposited materials. When the thickness of coated layer was about 20–30 μm and the deposited mixed metal salts and the CNT catalyst reached to around 5.5 mg/cm2, the repetition was enough. Schematic of electrode preparation was illustrated in Fig. S1.
2.4. Electrochemical Measurements
Electrochemical behavior of different Ti modified electrodes was studied by linear sweep voltammetry (LSV) technique. The LSV voltammograms were used to investigate the potential needed for water splitting in oxygen evolution and ozone generation. The LSV voltammograms were performed in a three-electrode system Ag/AgCl/KCl (3 M) reference electrode, platinum as cathode electrode and Ti or Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrodes as an anode) at 50 mV·s−1 in a solution containing HClO4 20 mL, 0.1 M at room temperature.
2.5. Electrochemical Ozone Generation and Measurement
Electrochemical ozone generation using the bare Ti and Ti/TiHx/SnO2-Sb2O5-NiO-CNT anode was performed in 4 mL of HClO4 (0.1 M) solution (two-electrode system; platinum was used as the counter electrode) by applying constant current density of 20 mA·cm−2 for 25 min. The amount of ozone gas generated by both electrodes were then measured according to the American Public Health Association (APHA) 4,500-O3 standard using ozone scavenger (potassium indigotrisulfonate at pH < 4.0) [66]. In this method, ozone concentration was calculated from changes in intensity light of the sample under electrolysis using the electrodes compared to a blank containing 1 mL HClO4 (0.1 M) and 3 mL potassium indigotrisulfonate ozone scavenger according to equation 1. The actual ozone generating sample was consisted of 1.0 mL HClO4 (0.1 M) and 3.0 mL of potassium indigotrisulfonate hosted the bare Ti and Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrodes for the electrolysis procedure. In Eq. (1), V is total volume (mL), ΔA is the absorption difference between the blank and the sample, f is a factor corresponding to an absorption coefficient for aqueous ozone at 2,950 M−1·cm−1 at 258 nm which is 0.42 (cm−1 per mg·L−1), b is path length of cell (cm) and v is volume of the sample (mL).
2.6. Dye Sample and Degradation Method
Initial concentration of 300 mg·L−1 of RR 195 in deionized water was used in all experiments to investigate the effect of electrolysis time on dye degradation, pH and COD changes of the solution. The dye degradation was performed in a two-electrode system, with Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode as anode and platinum as cathode in 4 mL of the dye solution by applying constant current density of 20 mA·cm−2 at 0, 5, 10, 15, 20, 25, 30, 35 and 40 min. Change at intensity of light absorbance and pH values were observed at different times and the COD was measured before and after the dye degradation procedure. Eq. (2) was used in order to evaluate the dye degradation efficiency at time t, where C0 is the initial concentration and Ct is the dye concentration at time t [8].
Calculation of COD was performed according to standard APHA 5,220-B method [66]. To investigate the effect of electrochemical degradation of dye on the COD of the solution, a sample containing 300 mg·L−1 RR 195 with the initial COD of 215 mg·L−1 O2 was prepared, then electrochemical ozon generation was done at the modified electrode. The initial COD of the dye solution and COD of the solution after 45 and 60 min of electrolysis were calculated using Eq. (3) and compared with initial concenteration. In this equation A is the consumed titrant for blank (mL), B is the volume of titrant used for the sample (mL) and M is molarity of titrant.
2.7. Kinetics of the Dye Degradation
The kinetics of the selected dyes for discoloration were studied. The Eq. (4) was used to calculate the constant rate and to investigate the kinetics of the dye degradation process, where dC/dt is the change in the dye concentration at different times, n is reaction kinetics order (0, 1 or 2) and k is rate constant of the dye degradation [67].
3. Results and Discussion
3.1. Parameter Optimization
The main propose of this study was the ozone generation in high efficiency on a stable electrode with adding TiHx sublayer. Prepared TiHx sublayer on Ti were done by Shao’s method [61]. Also, for fabrication of this electrode, the optimum values of metal salts of tin, antimony and nickel with molar ratio of 500:8:1 were used [4]. To optimize the carbon nanotubes value, five solutions with constant amounts of tin, antimony and nickel metal salts were prepared [4] and then carbon nanotubes was added to the solutions with molar ratio of 0, 5, 10, 15, 20 and 25 relative to Ni. For each electrode, the layering and thermal pyrolysis processes were performed three times. Then, the prepared electrodes were used as a working electrode in a two electrode system (the platinum electrode counter) by applying a current density of 20 mA·cm−2 during electrolysis (10 min) to generate ozone and the amounts of generated ozone on each electrodes were measured. Fig. S2 shows the optimization plot of the carbon nanotubes value. As shown, by increasing carbon nanotubes from the molar ratio of 0 to 15, the amount of generated ozone increases from 1.1 to 4.9 mg·L−1 and then it is nearly constant. Therefore, the molar ratio of 15 for carbon nanotubes was considered as an optimum value. The optimum molar ratio of metal salts and CNT (Sn:Sb:Ni:CNT) was obtained to be 500:8:1:15. The results show adding CNT to composite mixture can increase ozone generation more than two times.
Also, to ensure the complete coverage of the surface with a mixture of metal salts and carbon nanotubes, the number of electrode coatings based on the change of the electrode weight was performed. With increasing the number of repetitions of coating (from 1 to 22), weight of electrode increases (The weight of the electrode 3.61 mg increases for 22 times coating). When the thickness of coated layer was about 20–30 μm and the deposited mixed metal salts and the CNT catalyst reached to around 5.5 mg/cm2, the repetition was enough.
3.2. Characterization of Ti/TiHx/SnO2-Sb2O5-NiO-CNT Electrodes
SEM micrographs of bare and modified Ti electrodes are shown in Fig. 1. Fig. 1(a) indicates the surface of bare Ti after washing and etching with oxalic acid and Fig. 1(b) shows surface of the modified Ti electrode. As can be seen, the Ti electrode has a porous and rough surface while after modification; the pores are filled with nano catalysts and create a new surface with cracks which increases the effective surface area for higher and more efficient electron transfer to the solution.
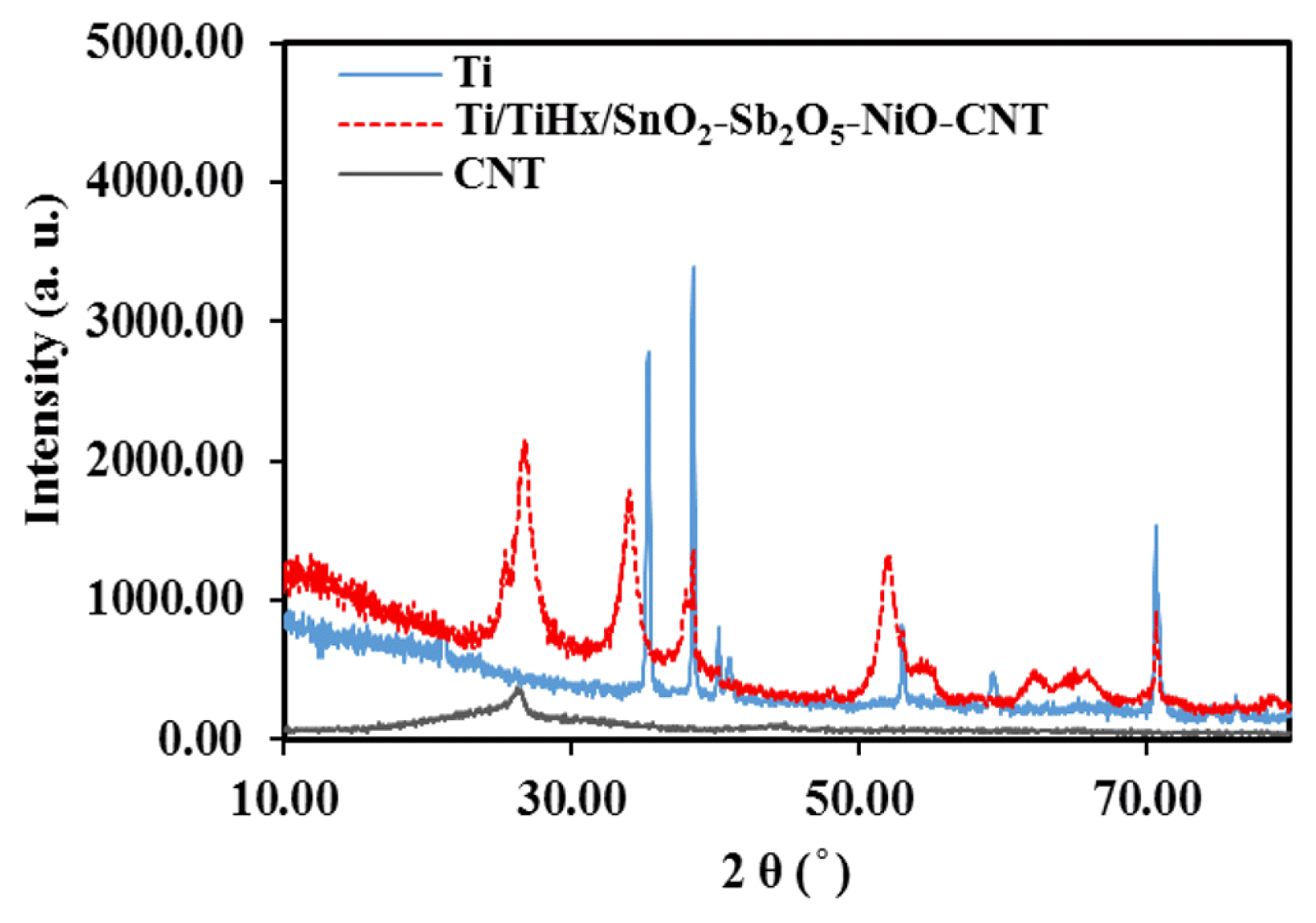
MMO crystalline phase formed on the electrode was investigated by XRD method. Fig. 2 shows the XRD pattern of CNT and Ti electrode before and after modification. It has been proved that the mixture of the metal oxides of tin, nickel, and antimony have are suitable anodic materials for ozone generation [1, 4, 5, 8, 53, 54]. Most designed electrodes for ozone generation are without sublayer so they have a short lifetime [55, 56]. Studies show that the addition of TiHx as a sublayer can increase the lifetime and stability of the electrode [60, 61]. In this research TiHx peaks appear at 2θ of 34.925, 40.340 and 70.896 deg. in the present spectrum confirms that the TiHx sublayer is properly formed (refer to ICDD 00-025-0983), although, some picks have overlap with Ti (refer to ICDD 00-005-0682). This results have a good agreement with reports from other researchers [60, 61]. Also, in the reported papers has also shown that the metal oxides of tin, antimony and nickel have good electrocatalytic properties for ozone production [1, 4, 5, 8, 53, 54]. In the present pattern, the related peaks appear at 2θ of 26.6, 33.929, 38.101, 52.203, 54.937, 62.260 and 79.074 deg. (main peaks: 26.6, 33.87 and 52.203) show tin dioxide was properly synthesised (refer to ICDD 00-001-0657). At 2θ of 26.873, 35.137, 38.168, 52.814 and 61.982 deg. show antimony pentoxide was also incorporated into the tin dioxide (refer to ICDD 00-050-1,376). Signals related to NiO base on (refer to COD 96-901-1,315) appear at 2θ of 33.128, 38.592, 52.114, 59.179, 62.837 and 70.434 deg. are not detected in this pattern, most likely due to the lowest molar content used to fabricate the anodes or overlaps with the tin oxide peaks [68]. This spectrum reveals that the metal oxides are formed well and has a suitable agreement with previous works [60, 61, 68]. Also, the spectrum of carbon nanotubes shows that the related peak appears at 2θ of 26.603 deg. overlaps with the tin oxide peak, although it increases its intensity. These results are in agreement with the results reported by other researchers and confirm them [9, 60, 61, 68]. The average size of crystals was also calculated as about 20 nm using the XRD patterns and the Scherrer Eq. (5).
Where L is the average crystal size of the nanoparticles (nm), K is Scherrer constant which is related to the morphology of nanoparticles (for spherical particles it was considered as 0.9), λ is the X-ray wavelength (nm), fwhm is half-height width of the peaks and θ is the degree of diffraction for each peak (Radian).
3.3. Electrochemical Behavior of the Electrodes
The greatest challenge in electrochemical ozone generation is its severely competition with oxygen evolution. Thermodynamically, water is much readily oxidized to oxygen relative to ozone (Eq. (6)). Electrochemical ozone generation is formed by electrolytic decomposition of water at the anode by Eq. (7).
Thus, the inhibition of oxygen evolution is a first requirement for an efficient generation of ozone. This can be achieved by suitable choice of the anodic material or the use of additives that partially inhibits the oxygen evolution reaction via blocking of its active sites [9].
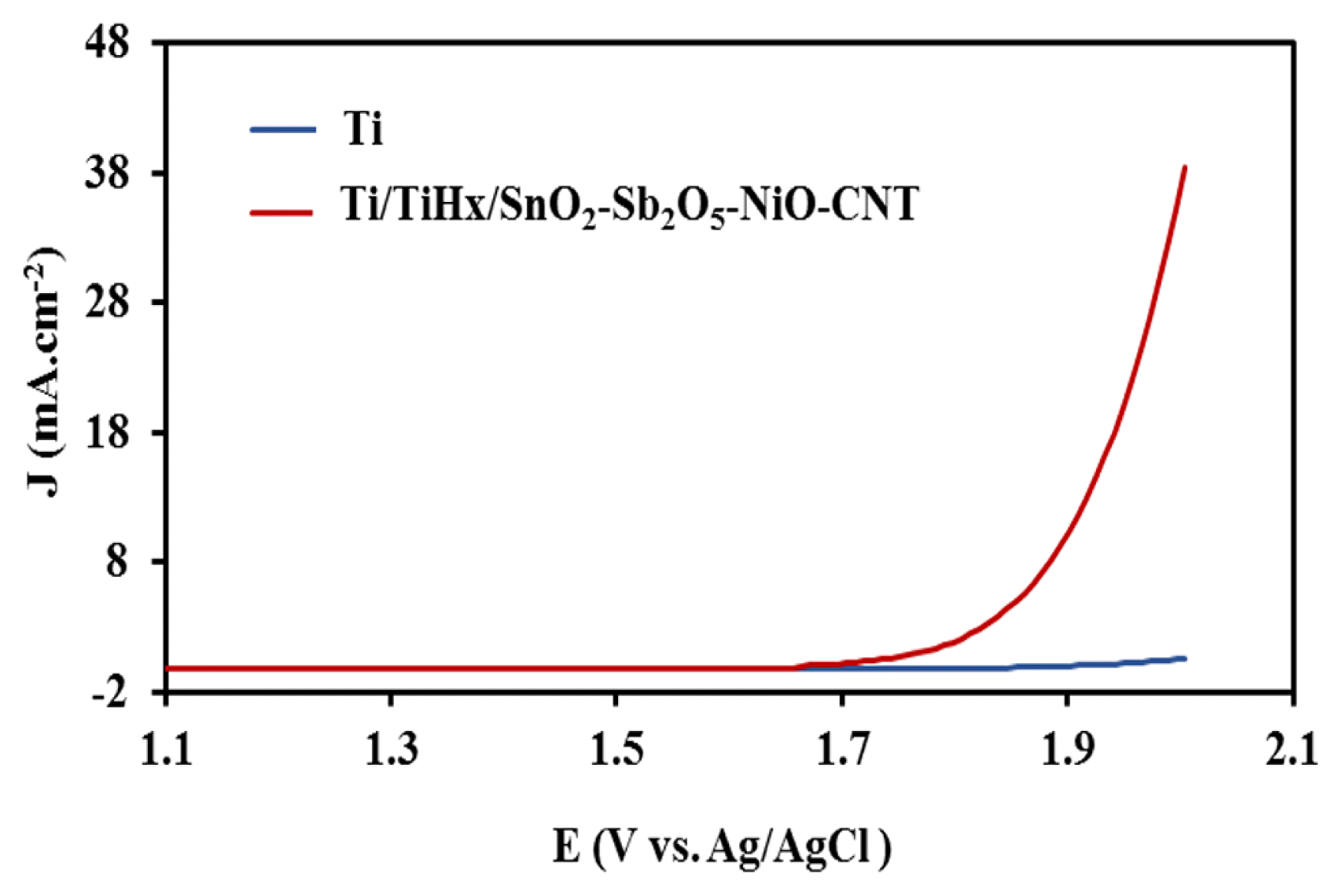
The LSV voltammograms of bare Ti and Ti/TiHx/SnO2-Sb2O5-NiO-CNT anode electrodes are shown in Fig. 3. The same of oxygen evolution at bare Ti electrode, an active surface coating is required for electrochemical ozone generation. After modifying the electrode, onset potential is 1.91 V vs. Ag/AgCl and it was much higher than the typical value for oxygen evolution in acid solution which is 1.01 V vs. Ag/AgCl, indicates that oxygen evolution was kinetically suppressed (see Eq. (6)). So, the results indicated the anodic current density of the modified Ti electrode compared to the bare Ti at a constant potential 2.0 V increased from 0.58 at bare Ti to 38.4 mA·cm−2 at Ti/TiHx/SnO2-Sb2O5-NiO-CNT. These voltammograms and interpretations show modified electrode as an selective electrode for electrochemical ozone generation. These results are in agreement with the results reported by other researchers and confirm them [9].
3.4. Ozone Generation and Measurement
The mechanism of ozone generation at Ti modified with tin, antimony and nickel oxides catalyst was studied and suggested by Gibson et al. [69] including the splitting of water to form oxygen and ultimately ozone through the interaction of oxygen and surface atomic oxygen.
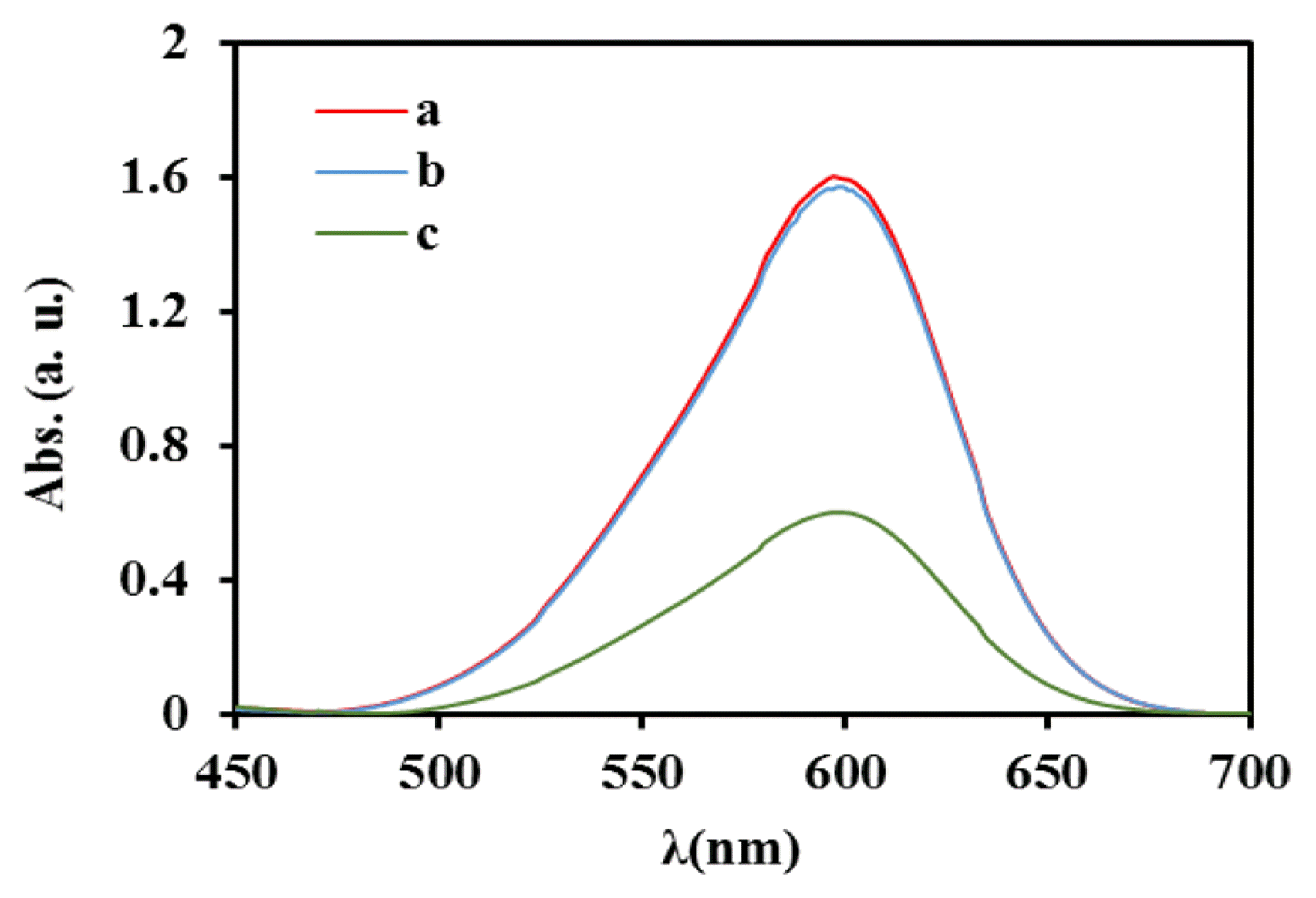
The electrochemical ozone generatied by bare Ti and modified Ti was measured with well known indigo method using potassium indigotrisulphonate based on the changes in light intensity absorbance of the ozone scavenger in electrolyte solution before and after electrolysis in presence of the electrodes [68, 70]. In Fig. 4 curves (a), (b) and (c) show the UV-Vis spectra of sample (ozone scavenger and HClO4 0.1 M) before electrolysis, after electrolysis at bare Ti electrode and at Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrodes, respectively. As this figure shows at bare Ti, ozone is generated slightly, so the absorbance of solution does not show significant change, while at Ti/TiHx/SnO2-Sb2O5-NiO-CNT a large amount ozone is genegated, so the absorbance at 600 nm shows a huge change. Based on the findings, after 25 min electrolysis at bare Ti and modified Ti electrode, respectively, the absorbance value for the indigo ozone scavenger at 600 nm decreases from 1.596 to 1.573 and 1.596 to 0.599, then dissolved ozone was calculated using Eq. (1). The results show at the same condition after electrolysis at bare Ti electrode as an anode the value of 0.2 mg·L−1 ozone was produced while at the modified Ti electrode the ozone value of 9.5 mg·L−1 was produced.
To have a clearer view of the advantages of using the modified Ti electrode fabricated in our research, the efficiency of ozone generation in our work is listed along with the values reported by other researchers in Table S3. According to the data, the efficiency of ozone generation in our novel electrode in this work, was better than some reports [4, 8, 10, 14] and worse than some other [9, 15].
3.5. Electrochemical Degradation of RR 195
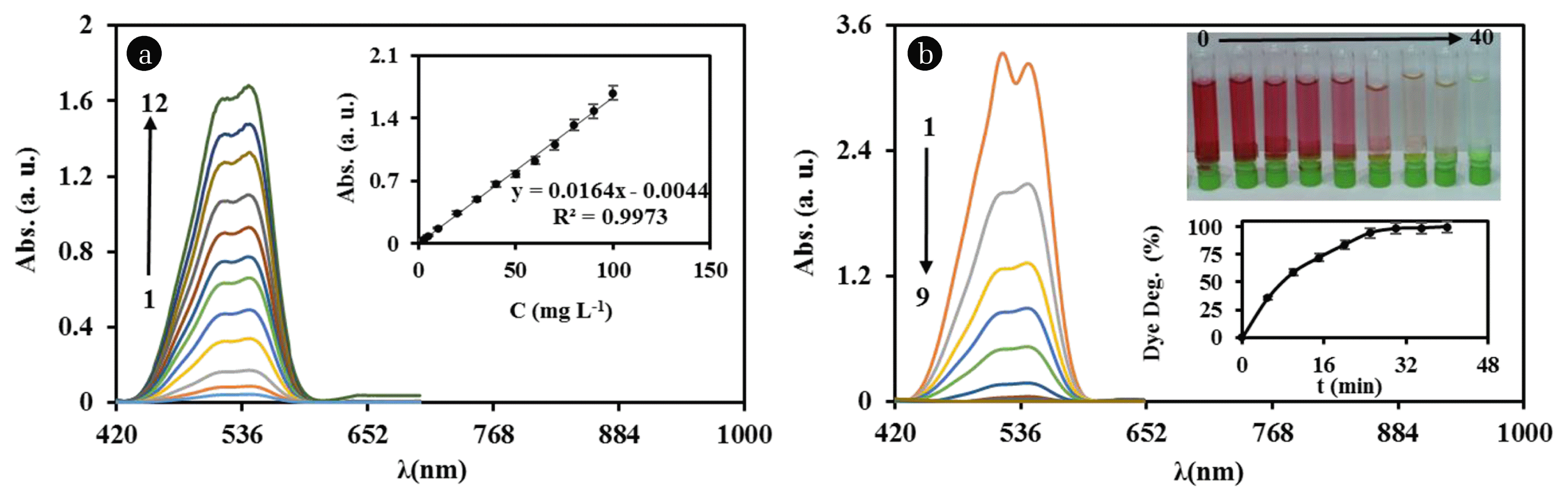
Electrochemical dye degradation for RR 195, was performed inside an electrochemical cell with a two-electrode arrangement, the Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode as anode and Platinum electrode as cathode at different time (0–40 min). To determine the dye concentration after electrochemical degradation process at time t, first, the absorption spectra of RR 195 were plotted in the different dye concentrations (2.5, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 ppm) (Fig. 5(a)). However, as displayed in Fig. 5((a) and (b)), the textile dye RR 195 revealed two characteristic absorption bands at 520 nm and 542 nm. A linear calibration curve was obtained from plotting the absorbance of dye solutions at 542 nm versus concentration, which is shown in inset of Fig. 5(a). The dye concentration during the degradation process at different time was estimated from this calibration curve. Experiments repeated 3 times in order to determine the standard error. Fig. 5(b) shows the UV-Vis absorbance spectra of RR 195 after degradation in presence of the Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode at different times. According to the figure, the absorbance value at 542 nm decreases from 3.14 to 0.10 with increasing the electrolysis time from 0 to 30 min. The inset of Fig. 5(b) shows the effect of electrolysis time on the efficiency of dye degradation. Increase in the electrolysis time could cause an increase in the ozone generation, and consequently increasing the dye degradation efficiency up to 30 min which raised to 96%. With increasing the time of electrolysis upto 40 min absorbance decreses from 0.1 to 0.0. Experiments repeated 3 times in order to determine the standard deviation.
3.6. Effect of Electrolysis Time on pH
Change in the pH of solution after the dye degradation in the presence of the Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode at different electrolysis times is shown in Fig. S3. As shown, by increasing the electrolysis time, pH of the solution is decreased from 4.13 to 3.02, which is due to the production of protons (H+) during the oxidation of water for ozone generation. It is also foresee able that the pH of solution may slightly decrease due to the oxidation of dye by ozone and production of acidic by-products. These experiments were also repeated 3 times.
3.7. Kinetics of RR 195 Degradation
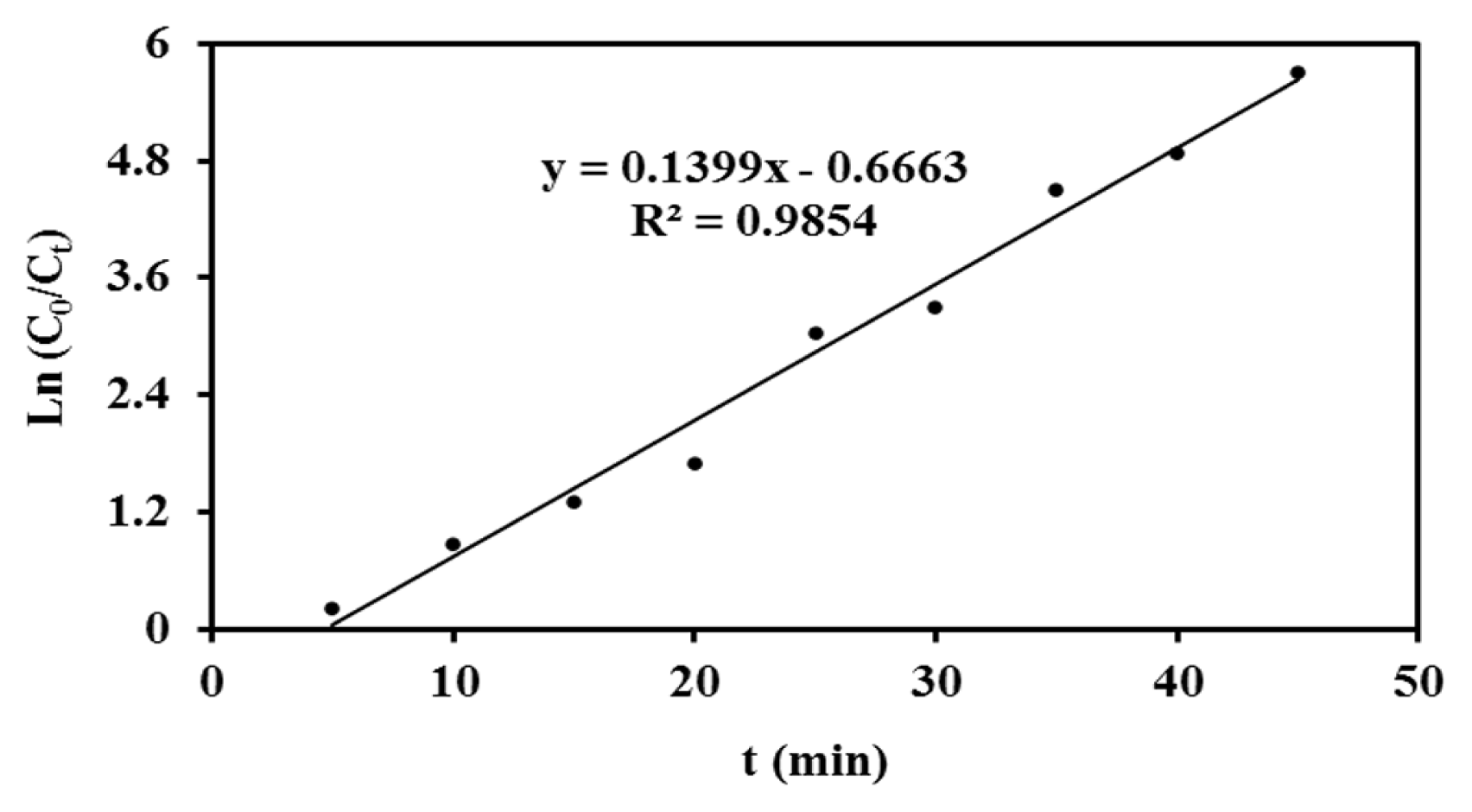
To study the kinetics of the dye degradation process, 300 mg·L−1 dye solution was prepared and then electrochemical dye degradation was performed in presence of the Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode at various times. The kinetic orders of zero, one and two were investigated using Eq. (4). Based on the values of the calculated correlation coefficient in each of these three kinetics models, it was concluded that the dye degradation process follows first-order kinetics (Table 1).
The value of constant rate of dye degradation (k) and correlation coefficient (R2) was determined from the slope of Ln (C0/Ct) vs t which is presented in Fig. 6.
3.8. COD Removal
The results revealed that the amount of COD of the dye solution reached to 130 mg·L−1 O2 after 45 min electrolysis which is a 40% decrease in COD. Decline in COD was directly related to the electrolysis time where it could reach to 60% reduction level in COD after 60 min. As it could be predicted the electrolysis process using the novel modified electrode can lead to dye degradation and COD removal which is highly necessary for wastewater treatment procedures.
To have a clearer view of the advantages of using the modified Ti electrode fabricated in our research, the efficiency in degradation of RR 195 observed in our work is listed along with the values reported by other researchers in Table 2. According to the data, our novel electrode can degrade RR 195 dye in a much more efficient way compared to the other reported works.
4. Conclusions
In this study, the electrochemical degradation of RR 195 dye, which is an example of a large group of toxic environmental pollutants, was investigated. A novel modified Ti/TiHx/SnO2-Sb2O5-NiO-CNT electrode was fabricated successfully and then characterized using SEM and XRD techniques. The designed electrode is capable of ozone generation as a green oxidizing agent by applying a constant current density of 20 mA·cm−2 which could reach a concentration of 9.5 mg·L−1 in the solution in 25 min electrolysis. The prepared electrode was able to degrade RR 195 dye molecules (300 mg·L−1) with the efficiency of 96% in 30 min electrolysis time with no need to other chemicals and without leaving any residual sludge as a pollutant to the environment. The degradation kinetics order of the dye (RR 195 dye) follows the first-order kinetics with a constant rate of 0.1399 min−1. Our newly presented Ti-modified electrode could be successfully used in electrochemical ozone generation with acceptable stability for destruction of colorants and other polluting agents in the advanced wastewater treatment procedures. Our modified electrode could be fabricated with a very low cost and its application in ozone generation will be a big leap forward in the constant fight of the responsible societies against the threatening environmental impact of polluting activities.