AbstractTo determine the grain size distribution and chemical characteristics of bottom surface sediments in shallow brackish lagoons, we studied sediment samples collected from the entire horizontal lake area and in vertical profiles from three stations in Lakes Youngrang and Hwajinpo, on the eastern coast of Korea. Vertical and horizontal grain size distributions of the bottom sediments indicated predominantly sand- and silt in both lakes. The vertical distribution of C/N ratios ranged from 6.14 to 11.92 in Lake Youngrang, and 6.74 to 12.34 in Lake Hwajinpo. The horizontal distribution of C/N ratios in Lake Youngrang ranged from 6.1 to 17.6, whereas they ranged from 4.4 to 12.1 in Lake Hwajinpo. C/N ratios showed locally different responses to the origin of allochthonous (partial region) and autochthonous (entire region) organic materials. Horizontally, bottom sediment with low δ13C and high δ15N in Lake Youngrang were likely to be influenced by autochthonous organic material derived from primary production, and would be affected by N inputs from sources. In contrast, high δ13C and low δ15N sediments in Lake Hwajinpo were likely to be influenced by cyanobacteria.
1. IntroductionThe environmental analysis of grain size distributions found in sedimentary deposits is an essential objective of sedimentology. The grain size of lake sediments has been commonly used to restructure environmental processes, and the several workings of lake sediments are influenced by different factors [1]. Grain size distribution is a key to understanding river processes and morphology, and for hydraulic, sediment transport, and fluvial habitat studies [2]. Sediments in the littoral zone are often resuspended by water currents and transported from the littoral area to the pelagic area [3]. Such horizontal sediment transport can affect the chemical and biological characteristics of both the water body and the sediment in the pelagic area.
The concentration of sedimentary organic carbon, nitrogen, C/N ratio, and isotopic composition (δ13C and δ15N) are among the most long-lived proxies of organic matter delivery and accumulation in lacustrine environments [4]. The C/N ratio is a common chemical characteristic of surface bottom sediments used to indicate the source of organic matter in aquatic sediments [5]. The C/N ratio in aquatic systems is controlled by the mixing of terrestrial and autochthonous organic matter [6–8]). Marine algae and particulate organic matter usually have C/N ratios < 10 [9], compared to C/N ratios > 12 for terrestrial C3 vegetation [10]. Carbon isotope ratios of organic matter have been also employed as source indicators of organic matter, because organic carbon in marine phytoplankton is often isotopically heavier than terrestrial organic carbon [11]. Nitrogen isotope ratios of sediments may also reflect the deposition rate of terrestrial organic matter, forms of nitrogen sources for phytoplankton, and decomposition processes, such as denitrification within the sediment [12]. Moreover, nitrogen isotopic signatures of sources and sinks of fixed nitrogen in sediment can be used to clarify marine nitrogen budgets [13].
The lagoons in the Korean Peninsula are mostly dispersed in the eastern coastline. Among the 57 natural lakes in this region 48 of them are marine origin natural lakes which were formed by sand dunes due to the ocean water currents [14, 15]. There are 12 lagoons on the east coast of Korea, including Kyeongpo, Hyang, Mae, Chungcho, Youngrang, Songji, and Hwajinpo lakes. These lakes are not only historically important, but they have high geological and ecological value [16]. Among these lagoons, Lake Hwajinpo, characterized as a relatively natural lake with little human impact, has a natural opening (natural seawater inflow). The north basin of Lake Hwajinpo has a narrow inlet to the sea that is connected only during the summer rainy season or by seawater inflow due to large waves. Conversely, Lake Youngrang is affected by discharge from septic systems near the lake, and is semi-open (artificial seawater inflow). The lake was almost completely isolated from the sea by sand barriers prior to 2006, and formed a very low saline water mass within the closed area. After a temporal and artificial opening event (high saline water mass) in 2006, numerous freshwater fish (Cyprinus carpio) migrated upstream and were observed in surface layers. Since then, the lake has frequently been artificially opened because a sand barrier has been slowly forming due to longshore drift.
In this study, the vertical and horizontal distribution characteristics of Lakes Youngrang (artificial opening) and Hwajinpo (natural opening) were studied, using C/N ratios, δ13C and δ15N stable isotope values, and grain size of bottom sediment. The aim of this study was to estimate the vertical and horizontal variability of these parameters, and the origin of autochthonous and allochthonous organic matter within deposited sediment in two different lakes.
2. Materials and methods2.1. Study SitesThis study was conducted in two lagoons located on the east coast of Korea (Fig. 1). Lake Youngrang has a surface area of 0.96 km2, comprising paddy fields (1.11 km2) and forest areas (3.67 km2), with a maximum depth of 6.2 m (Fig. 2), similar to that described by Cho and Park [17], and is affected by discharge from septic systems near the lake.
Lake Hwajinpo has a surface area of 2.06 km2, comprising paddy fields (5.17 km2) and forest areas (10.97 km2) with a maximum depth of 4.2 m in the north basin and 3.3 m in the south basin. The lake becomes gradually deeper from south to north, and the greatest depth was measured in the center in the north basin (Fig. 2).
Salinity is lower in Lake Hwajinpo (13.7 ± 2.1 ppt) than in Lake Youngrang (19.6 ± 5.4 ppt). N loading (kg day−1 km−2) is 3.8 in Lake Hwajinpo and 17.6 in Lake Youngrang [18].
2.2. Sample Collection and PreparationTo analyze vertical distributions of parameters, samples were taken from the upper 10 cm of bottom sediments, at 1 cm intervals, from three stations (Sta. A, B, and C) in each lake using a core sampler. To analyze horizontal distributions, bottom sediments were collected over 20 cm depths from the entire area of both lakes at regular intervals (Lake Youngrang: 37 stations, Lake Hwajinpo: 47 stations) using an Ekman-Birge dredge. Sampling was conducted in April to October 2009. Wet sediments were stored in a refrigerator prior to grain-size analysis. Particle size was measured by a Laser Diffraction Particle Size Analyzer (Shimadzu, SALD-2000J). Previous records of the dredging history and watershed changes over the past 50 years were obtained from the regional government office (Sokcho City Hall).
Organic carbon and nitrogen were determined with a CHN Corder (Yanaco, MT-5) after carbonate carbon was removed from the sediment using 1M HCl solution (Note that there is little difference between samples treated with 1M HCl and untreated samples; Student’s t-text, P > 0.05). The isotopic composition of carbon and nitrogen in the samples was determined by a stable-isotope analyzer (ANCA 20–20; Europa Scientific) with a preparation system for solid and liquid samples (ANCA-SL; Europa Scientific). The standard deviation of the isotopic analysis was less than 0.1 ‰ and 0.3 ‰ for δ13C and δ15N, respectively.
2.3. Statistical AnalysesData were analyzed using one-way analysis of variance (ANOVA). Statistical analyses were conducted using SigmaPlot 11 and results were considered statistically significantly when p<0.05. To describe the distribution of environmental data in the lakes and stations, we used a principle component analysis (PCA).
3. Results and Discussion3.1. Vertical and Horizontal Distribution of Grain Size in Bottom SedimentsThe bottom sediments of both lakes mainly consist of sandy-silt and clayey-silt. Lake Youngrang shows a relatively larger grain size than Lake Hwajinpo (Fig. 3). Regarding the vertical distribution of grain size at each station, the median grain-size diameter ranges from 4.3μm (clayey-silt size; Sta. A) to 19.9 μm (silt-sand size; Sta. A) in Lake Youngrang, and from 13.4 μm (silt-sand size; Sta. C) to 202.2 μm (sand-size; Sta. A) in Lake Hwajinpo. No significant difference in grain size distribution is observed between stations (one-way ANOVA: F = 1.5, P > 0.05). However, much larger grain sizes are observed in the uppermost layer of Sta. C in Lake Youngrang (Table 1), suggesting the possibility of lateral transportation from elsewhere by lake currents and inflow from the sea. Kumon et al. [19] reported that the inner zone of a concentrically circular lake has finer grain sizes, and that the center slightly larger grain sizes than the surrounding area. Our results suggest that Sta. C in Lake Youngrang might be influenced by lake currents and the lake center, similar to the findings of Cho and Park [17].
Regarding the deposition rate, however, Nobuyuki et al. [20] reported that sedimentation rates in Lake Youngrang are approximately 1.52 mm/year (Lake Hwajinpo, ≈ 1.64 mm/year; [21]), calculated as 152 cm/1,000 years. Our data suggests that Sta. C in Lake Youngrang may be highly influenced by the surrounding conditions due to its much larger grain size (120.7 μm) between 0 and 1 cm. This may be because between 2001 and 2008, deposition was mostly caused by sediment dredging (Sokcho City Hall record) or inflow from the sea.
Moreover, relatively larger grain sizes (19.4 μm) between 5 and 6 cm are assumed from 1968 to 1974, indicating that Lake Youngrang might have been influenced by the surrounding environment through construction of reclaimed land, golf courses, and housing complex. In Lake Hwajinpo, grain size is quite different among stations (Table 2; one-way ANOVA: F = 73.1, P < 0.05), and much larger grain sizes are observed at Sta. A, suggesting that it is supplied by a tributary stream, and sediment may be transported in suspension under the influence of wave action (Tables 1, 2).
The horizontal grain size distributions of both lakes are shown in Fig. 3. The median diameter ranges from 7.9 to 63.5 μm in Lake Youngrang and 10.9 to 60.0 μm in Lake Hwajinpo. The two lakes show no significant differences in grain size (Student t-test P > 0.05). The bottom sediments predominantly consist of silt. Much larger grain sizes are observed in Lake Youngrang at stations close to the sea or artificial islands. In Lake Hwajinpo, larger grain sizes are observed at stations near the sea, and where water is flowing into streams and around bridges at the boundary between north and south basins. Large sand near the bridges between basins is probably due to the surrounding construction, irrespective of organic matter, microorganisms, and humic acid content [24]. Moreover, the shoreline areas in each lake showed significantly larger grain sizes. This can be explained by a lower sampling density near the shoreline.
Heo et al. [23] reported that the surface sediments in Lakes Hyangho and Kyungpo mostly consist of silt and silty sand, respectively. In our results, the dominant compositions are clayey silt in Lake Youngrang and sandy silt in Lake Hwajnpo (Fig. 3). This suggests that grain size varies largely between brackish lakes because of variable deposition of organic matter, erosion, dead plant debris, aquatic plants, sand microorganisms [23], and watershed environments.
Furthermore, our study showed that the center area is slightly smaller grain size than the surrounding area (Fig. 4), indicating that grain size decrease from surrounding area to central area. We suggest that median grain size values might differ toward the center of lake, in that smaller grain sizes might be decomposed and deposited after lateral transport into deeper water by lake currents.
3.2. Vertical and Horizontal Distributions of C/N Ratio
Tables 1–2 show the vertical distribution of carbon and nitrogen contents in the bottom sediments of the two lakes. The C/N ratio vertical profile ranges from 6.14 to 11.92 in Lake Youngrang (Table 1), and from 6.74 to 13.42 in Lake Hwajinpo (Table 2). These values suggest that organic matter is predominantly planktonic in origin [5]. The C/N ratios of the vertical profiles at Sta. B and C in Lake Youngrang indicate a sedimentary layer and autochthonous organic material derived from primary production, while the 3 to 5 cm layer at Sta. A, which includes a major inflowing stream, might contain more allochthonous organic matter originating from the watershed. The C/N ratio at Sta. C in Lake Youngrang shows an abrupt increase in the layer (4–6 cm). These results suggest that the sediments at Sta. C, characterized by deep water, are richer in allochthonous and refractory organic material than other stations [24]. The C/N ratios of the vertical profile at Sta. A in Lake Hwajinpo are high, suggesting an influence of the tributary stream on the bottom sediment.
Fig. 5 shows the horizontal distribution of carbon and nitrogen contents in the bottom sediments of the lakes. The bottom sediment in the littoral zone of both lakes is lower in carbon and nitrogen than that in the profundal zone. This result is similar to that reported by Murase et al. [24]. The carbon and nitrogen contents of the bottom sediment were relatively higher toward the central area of the lake, suggesting the lateral transport of sedimentary organic matter from the littoral zone to the profundal zone by lake currents [24].
The C/N ratio of bottom sediments is often used as an important indicator of the origin of organic matter in lacustrine depositional environments. That is, the primary nitrogen components of phytoplankton and zooplankton have C/N ratios of 5 to 6 [25, 26], freshly deposited organic material originating from planktonic organisms has C/N ratio of 6 to 9 [10, 25, 27], and organic materials derived from terrestrial vascular plants have C/N ratios of > 15 [26, 28–32]. In our study, the C/N ratios show similar spatial variations, ranging from 6.1 to 17.6 in Lake Youngrang, and from 4.7 to 12.1 in Lake Hwajinpo. The two lakes show significantly differences (Student’s t-test, P < 0.01).
The C/N ratio in Lake Youngrang has a negative correlation with water depth (Fig. 5a), but a positive correlation in both north and south basins of Lake Hwajinpo (Fig. 5b). This may reflect the increased or decreased influence of aquatic macrophytes in each lake with water depth. These results indicate depletion in the C/N ratio of Lake Youngrang with water depth, and the converse in Lake Hwajinpo.
Regarding local of the C/N ratio in each lake, a concave area in the south (Y28 to Y31) and the west basins (Y1 to Y10) in Lake Youngrang exhibit higher C/N ratios than other stations. We suggest that high ratios in the western basin, which has a major inflowing stream and is well vegetated, may reflect the contribution of allochthonous organic material from Jang-stream and from nearby aquatic macrophytes. The concave southern area might indicate a dominant supply of allochthonous organic material from the watershed, and lake currents may be impeded due to the narrow, concave area. In contrast, C/N ratios of the north basin in Lake Hwajinpo are higher than in the south basin, characterized by a eutrophic state. This may be due to aquatic macrophyte development on the shore, and input of carbon from a small, unnamed stream.
According to Heo et al. [22], the north basin has a low nutrient concentration from the effects of seawater, and is a minorly eutrophic lake relative to the south basin. Conversely, the south basin has better conditions for plankton growth because of high nutrient concentrations and minimal effects from seawater. Indeed, C/N ratios in Lake Hwajinpo reveal significant differences between the north and south basins (Student’s t-test, P < 0.01). These facts suggest that the relatively high C/N ratios of bottom sediments observed in the north basin (H1 to H13) can be interpreted as the organic carbon content of the bottom sediments derived from allochthonous sources under the influence of well-developed aquatic macrophytes. Conversely, relatively low C/N ratios observed in the south basin (H14 to H47) in Lake Hwajinpo are due to high contents of the primary nitrogen compounds of phytoplankton and zooplankton, which have low C/N values due to the accumulation of sedimentary planktonic organisms.
3.3. Distribution of Carbon and Nitrogen Stable IsotopesSediment δ13C values in Lake Youngrang vary from −25.3 to −20.2 ‰ (−23.4 ± 1.3 ‰, mean ± SD), and from −25.3 to −20.2 ‰ (−22.0 ± 1.0 ‰) in Lake Hwjinpo. Sediment δ13C values in Lake Youngrang are lower than those in Lake Hwajinpo (P < 0.001), and most stations in Lake Youngrang have lower δ13C contents than Lake Hwajinpo. Umemura et al. [33] reported that dissolved methane concentrations were lower in Lake Hwajinpo than those of Lake Youngrang. The methane produced in anaerobic lake sediments can have low δ13C values as a consequence of carbon isotope fractionation during its production by methanogenic archaea [34]. Furthermore, Woodward et al. [35] proposed that methane production in anaerobic lake sediments is more important for driving lake sediment δ13C values than algal production alone.
However, our results are likely to be influenced by the fact that cyanobacteria (Anabaena spp.) are the dominant phytoplankton in Lake Hwajinpo, which exposed to frequent cyanobacterial blooms with high density (2.3×104 cells mL−1) compared to several eutrophic lakes[36], during the summer season, whereas the phytoplankton community in Lake Youngrang is often dominated by Asterococcus limneticus [18].
Cyanobacteria are capable of active CO2 transport and utilizing HCO3− [37], and both can result in higher δ13C values in a phytoplankton biomass. High δ13C DIC (dissolved inorganic carbon) in the water column, with high demand for inorganic carbon due to high primary productivity, produces autochthonous organic matter with high δ13C values, which is then deposited in the sediments [38].
Yamamuro [39] reported that sediment δ13C shows a negative correlation with distance from the seawater entrance. That is, higher δ13C values are observed with increasing salinity along numerous estuaries [40, 41]. Their results are similar to ours, indicating that ours are also affected by seawater, assuming that the δ13C of DIC supplied from seawater has a higher value than that of freshwater in a brackish mixture [42]. In our study, high δ13C values are found near stations influenced by seawater, suggesting that stations were indeed affected by distance from the seawater intrusion. For example, Lake Youngrang shows higher values at stations Y22 to Y26 and Y32 to Y37 (mean −22.9‰) than other stations (mean −23.6‰), and Lake Hwajinpo has higher values at north basin stations (H1 to H14; mean −21.6‰) than south basin stations (H15 to H47; mean −22.2‰).
The principal component analysis (PCA) results show the grouping of stations in both lakes (Fig. 6b). Sediment δ15N values in Lake Hwajinpo are lower than those in Lake Youngrang. Sediment δ15N values range from 0.7 to 8.7‰ (6.1 ± 2.0‰, mean ± SD) in Lake Youngrang, and from 1.1 to 8.3‰ (4.4 ± 1.1‰) in Lake Hwajinpo, the latter of which are approximately 2‰ lower (Fig. 6a, P < 0.001). The additional gradient of δ15N and water depth factors can be attributed to the greater isotopic ratio of δ15N with increasing water depth in Lake Youngrang (center area stations; approximately Y11 to Y30), suggesting a strong influence of water depth on sediment δ15N. In our study, the higher δ15N values in Lake Youngrang than in Lake Hwajinpo might be due to differences in N sources from the watersheds [43–46]. N sources from human and animal waste typically have higher δ15N values than other N sources [46, 47]. Lake Youngrang receives N inputs from sources such as waste water and agricultural runoff [46], in contrast to Lake Hwajinpo. However, the low δ15N values (average 4.4 ‰) in Lake Hwajinpo are interpreted to be caused by the input of N fixed by cyanobacteria. Lake Hwajinpo only has blooms of Anabaena sp., unlike Lake Youngrang [18, 46]. N-fixing algae such as Anabaena sp. often have lower δ15N values, ranging from approximately −2 to +2 [49–51].
When δ13C and δ15N values are plotted against each station in Fig. 6(a), there is good separation among the sediments from both lakes. Sediment δ13C and δ15N values in Lake Youngrang show various spatial variations compared with Lake Hwajinpo (Fig. 6), suggesting that Lake Hwajinpo has relatively well-preserved natural sedimentary records compared to other lagoons in Korea [21, 52], and has stable sediment because the between site variability in δ13C and δ15N is small. Scattered δ13C and δ15N values in Lake Youngrang are likely to be the result of various anthropogenic impacts such as inflows of N sources and the salinity gradient caused by the artificial opening. However, the influence of saline water prior to the artificial opening would have been very small, even if the present Lake Youngrang is highly affected by seawater.
In conclusion, Lake Youngrang contains horizontal bottom sediment with low δ13C and high δ15N values influenced by autochthonous organic material derived from primary production, and is likely affected by N inputs from sources such as waste water and agricultural runoff. In contrast, high δ13C and low δ15N values in Lake Hwajinpo are likely influenced by the fact that cyanobacteria (Anabaena spp.) are the dominant phytoplankton. Furthermore, the δ13C values of dissolved inorganic carbon decrease in proportion of the amount of freshwater in a brackish mixture [42], according to salinity concentrations.
4. ConclusionOur study has shown that the vertical and horizontal distribution of grain size in bottom sediments differs between two brackish lakes and stations within the lakes. Bottom sediments in both lakes mainly comprise sand and silt. Carbon and nitrogen contents tend to increase with increasing water depth, and C/N ratios show local differences in response to the origin of allochthonous (partial region) or autochthonous (entire region) organic materials.
A horizontal distribution of sediment with high δ13C and low δ15N occurs in Lake Hwajinpo, whereas the opposite is the case for Lake Youngrang. This is likely the result of a nitrogen-fixing cyanobacteria (Anabaena spp.) bloom that only occurred in Lake Hwajinpo. The bottom sediments in Lakes Youngrang and Hwajinpo are influenced by complex environmental factors such as cyanobacterial, salinity concentration, N-sources, and water depth.
AcknowledgementThe authors wish to thank the laboratory members of School of Environmental Sciences, University of Shiga Prefecture, for their generous assistance in the chemical analyses and in the filed investigations. This study was supported by 2020 the RDA Fellowship Program of Organic Agriculture Division, Rural Development Administration, Republic of Korea.
NotesAuthor Contributions I.S. (Ph.D) checked all experimental results and wrote a manuscript. T.A. (Ph.D), H.A. (Ph.D), L.A. (Ph.D student), N.A. (M.D), M.O. (Ph.D) conducted all the experiments, S.L. (M.S) and S.Y. (M.S) conducted results analysis. N.I. (Professor), N.G. (Professor), M.M. (Professor), A.Y. (Professor), Y.S. (Professor), J.C. (Professor), Y.B. (Ph.D), B.L. (Ph.D) supported writing manuscript. O.M. (Professor) and K.C. (Ph.D) approved all experimental results and modified the manuscript. References1. Huang X, Sun M, Xiang L, Zhang E, Zhang J, Grimm EC. The effect of diatoms on the grain size of lake sediments: a case study of the sediments of Lake Kanas. J Paleolimnol. 2020;63:101–111.
2. Verdú JM, Batalla RJ, Martinez-Casasnovas JA. High-resolution grain-size characterization of gravel bars using imagery analysis and geo-statistics. Geomorphlogy. 2005;71:73–93.
3. Morikawa H, Okubo K, Muramoto Y. Intrusion of turbid waters in stratified lakes driven by resuspension of sediments on sloping boundaries. Annual J Hydrol Eng JSC (Mizukogaku-ronbunshu). 1996;40:607–612.
4. Contreras S, Werne JP, Araneda A, Urrutia R, Conejero CA. Organic matter geochemical signatures (TOC, TN, C/N ratio, δ13C and δ15N) of surface sediment from lakes distributed along a climatological gradient on the western side of the southern Andes. Sci Total Environ. 2018;630:878–888.
5. Sampei Y, Eiji M. C/N ratios in a sediment core from Nakaumi Lagoon, southwest Japan–usefulness as an organic source indicator. Geochem J. 2001;35:189–205.
6. Meyers PA. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org Geochem. 1997;27:213–250.
7. Thornton SF, McManus J. Application of organic carbon and nitrogen stable isotope and C/N ratios as source indicators of organic matter provenance in estuarine systems: evidence from the Tay Estuary, Scotland. Estuarine Coastal shelf Sci. 1994;38:219–233.
8. Zhao Y, Wu F, Fang X, Yang Y. Topsoil C/N ratios in the Qilian Mountains area: Implications for the use of subaqueous sediment C/N ratios in paleo-environmental reconstructions to indicate organic sources. Palaeogeogr Palaeclimatol Palaeoecol. 2015;126:1–9.
9. Meyer PA. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem Geol. 1994;114:289–302.
10. Prahl FG, Bennett JT, Carpenter R. The early diagenesis of aliphatic hydrocarbons and organic matter in sedimentary particulates from Dabob Bay, Washington. Geochim Cosmochim Acta. 1980;44:1967–1976.
11. Fry B, Sherr EB. δ13C measurements as indicators of carbon flow in marine and freshwater exosystems. Contrib Mar Sci. 1984;27:13–47.
12. Mishima Y, Hoshika A, Tanimoto T. Deposition rates of terrestrial and marine organic carbon in the Osaka Bay, Seto Inland Sea, Japan, determined using carbon and nitrogen stable isotope ratios in the sediment. J Oceanogr. 1999;55:1–11.
13. Rooze J, Meile C. The effect of redox conditions and bioirrigation on nitrogen isotope fractionation in marine sediments. Geochem Cosmochim Acta. 2016;184:227–239.
14. Lee MB, Kim NS, Lee GR. The distribution and geomorphic changes of natural lakes in east coast of Korea. J Kor Associat Region Geograph. 2006;12:449–460.
15. Bhattrai BD, Kwak SJ, Choi KS, Heo WM. Assessment of long-term physicochemical water quality variations by PCA technique in Lake Hwajinpo south Korea. J Environ Prot. 2017;8:1636–1651.
16. Choi JK, Mitamura O, Seike Y, Fujinaga K. Fish fauna of the Hwanipo Lake, Korea. Acta Hydrobiol Sinica. 2006;30:633–637.
17. Cho KS, Park YS. Limnological studies of the Youngnang lake. Kor J Limnol. 1968;2:51–66.
18. Wonju Regional Environmental Office. 2008. Lagoon Ecosystem Restoration Project Recommendation for Restoration and Management. Chuncheon: Kangwon National University, Korea.;
19. Kumon F, Kamitani T, Sutoh K, Inouchi Y. Grain size distribution of the surface sediments in Lake Biwa, Japan. Mem Geol Soc Japan. 1993;39:53–60.
20. Nobuyuki N, Hong SU. Paleclimatic features were examined by the geochemical method with sediments from Lake Yonglang in Korea. Kor J Limnol. 1982;15:13–18.
21. Yum JG. Characteristics of a coastal lagoon, Hwjinpo, in the eastern coast of Korea and its comparison with coastal lagoons in Sannin region in Japan [dissertation]. Shimane: Univ. of Shimane; 1996.
22. Heo WM, Kwon SY, Lee JI, Kim DJ, Kim BC. The Limnological Survey of a Coastal Lagoon in Korea (3): Lake Hwajinpo. Kor J Limnol. 2004a;37(1)12–25.
23. Heo WM, Kwon SY, Lee JI. The Limnological survey of a coastal lagoon in Korea (2): Lake Hyangho. Kor J Limnol. 2004b;37(1)1–11.
24. Murase J, Sakamoto M. Horizontal distribution of carbon and nitrogen and their isotopic compostions in the surface sediment of Lake Biwa. J Soc Limnol. 2000;1:177–184.
26. Bordowskiy OK. Accumulation of organic matter in bottom sediments. Marine Geology. 1965b;3:33–82.
27. Biggs RB, Sharp JH, Church TM, Tramontano JM. Optical properties, suspended sediments, and chemistry associated with the turbidity maxima of the Delaware Estuary. Can J Fish Aquat Sci. 1983;40:172–179.
28. Ertel JR, Hedges JI. The lignin component of humic substances: Distribution among soil and sedimentary humic, fulvic, and base-insoluble fractions. Geochim Cosmochim Acta. 1984;48:2065–2074.
29. Post WM, Pastor J, Zinke PJ, Stangenberger AG. Global patterns of soil nitrogen storage. Nature. 1985;317:623–616.
30. Ertel JR, Hedges JI, Devol AH, Richey JE. Dissolved humic substances of the Amazon River system. Limnol Oceanogr. 1986;31:739–754.
31. Hedges JI, Clark WA, Quary PD, Richihey JE, Devol AH, Santos UDM. Compositions and fluxes of particulate organic material in the Amazon River. Limnol Oceanogr. 1986;31:717–738.
32. Orem WH, Burnett WC, Landing WM, Lyons WB, Showers W. Jellyfish Lake, Palau: Early diagenesis of organic matter in sediments of an anoxic marine Lake. Limnol Oceanogr. 1991;36:526–543.
33. Umemura M, Yokoyama A, Akatuska T, et al. Dynamics of dissolved and bubbled methane in Lake Youngrang and Hwajinpo, Korea. Rep Res Ctr Inlandwat Environ. 2000;6:69–72.
34. Whitical MJ. Carbon and hydrogen isotope systematic of bacterial formation and oxidation of methane. Chem Geol. 1999;161:291–314.
35. Woodward CA, Potito AP, Beilman DW. Carbon and nitrogen stable isotope ratios in surface sediments from lakes of western Ireland: implications for inferring past lake productivity and nitrogen loading. J Paleolimnol. 2012;47:167–184.
36. Kim HS, Kim BC, Choi EM, Hwang SJ. Effects of Cyanobacterial bloom on zooplankton community dynamics in several eutrophic lakes. Korean J Limnol. 2000;33:366–373.
37. Espie GS, Miller AG, Kandasamy RA, Canvin DT. Active HCO3-transport in cyanobacteria. Can J Bot. 1991;69:936–944.
38. Torres IC, Ingleet PW, Brenner M, Kenney WF, Reddy KR. Stable isotope (δ13C and δ15N) values of sediment organic matter in subtropical lakes of different trophic status. J Paleolimnol. 2012;47:693–706.
39. Yamamuro M. Chemical tracers of sediment organic matter origins in two coastal lagoons. J Mar Sys. 2000;26:127–134.
40. Wilson GP, Lamb AL, Leng MJ, Gonzalez S, Huddart D. Variability of organic δ13C and C/N in the Mersey Estuary, UK and its implications for sea level reconstruction studies. Estuarine Coastal shelf Sci. 2005;64:685–698.
41. Middelburg JJ, Herman PMJ. Organic matter processing in tidal estuaries. Mar Chem. 2007;106:127–147.
42. Coffin RB, Cifuentes LA, Elderidge PM. The use of stable carbon isotopes to study microbial processes in estuaries. Washington, DC: U.S. Environmental Protection Agency; 1994.
43. Cabana G, Rasmussen JB. Comparison of aquatic food chains using nitrogen isotopes. Proc Nat Acad Sci USA. 1996;93:10844–10847.
44. Choi WJ, Han GH, Lee SM, Lee GT, Yoon KS, Choi SM, Ro HM. Impact of land-use types on nitrate concentration and δ15N in unconfined groundwater in rural areas of Korea. Agric Ecosys Environ. 2007;120:259–268.
45. Xu J, Xie P, Zhang M, Zhou Q, Zhang L, Wen Z, Cao T. Icefish (Salangidae) as an indicator of anthropogenic pollution in freshwater systems using nitrogen isotope analysis. Bull Environ Contamin Toxicol. 2007;79:323–326.
46. Lee JY, Choi JS, Owen JS, Lee KY, Heo WM, Kim BC. Habitat-specific variation in stable C and N isotope ratios of pond smelt (Hypomesus nipponensis). Anim Cells Syst. 2013;17:213–219.
47. McClelland JW, Valiela I, Michener RH. Nitrogenstable isotope signatures in estuarine food webs: A record of increasing urbanization in coastal watersheds. Limnol Oceanogr. 1997;42:930–937.
48. McClelland JW, Valiela I. Linking nitrogen in estuarine producers to land-derived sources. Limnol Oceanogr. 1998;43:577–587.
49. Gu B, Alexander V. Estimation of N2 fixation based on differences in the natural abundance of 15N among freshwater N2-fixing and non-N2-fixing algae. Oecologia. 1993;96:43–48.
50. Yoshioka T, Wada E. A stable isotope study on seasonal food web dynamics in a eutrophic lake. Ecology. 1994;75:834–846.
51. Vuorio K, Meili M, Sarvala J. Taxon-specific variation in the stable isotopic signatures (δ13C and low δ15N) of lake phytoplankton. Freshwater Biol. 2006;51:807–822.
52. Park BK, Kim WH. The depositional environments of lagoons in the east coast of Korea. J Geol Soc Korean. 1981;17:241–249.
Fig. 1Map showing the study areas of Lakes Youngrang ((a) and (b)) and Hwajinpo (c) and (d). (a) and (b) show vertical measurement stations, and (b) and (d) show horizontal measurement stations in each lake. “Y” indicates a station in Lake Youngrang, and “H” indicates a station in Lake Hwajinpo. 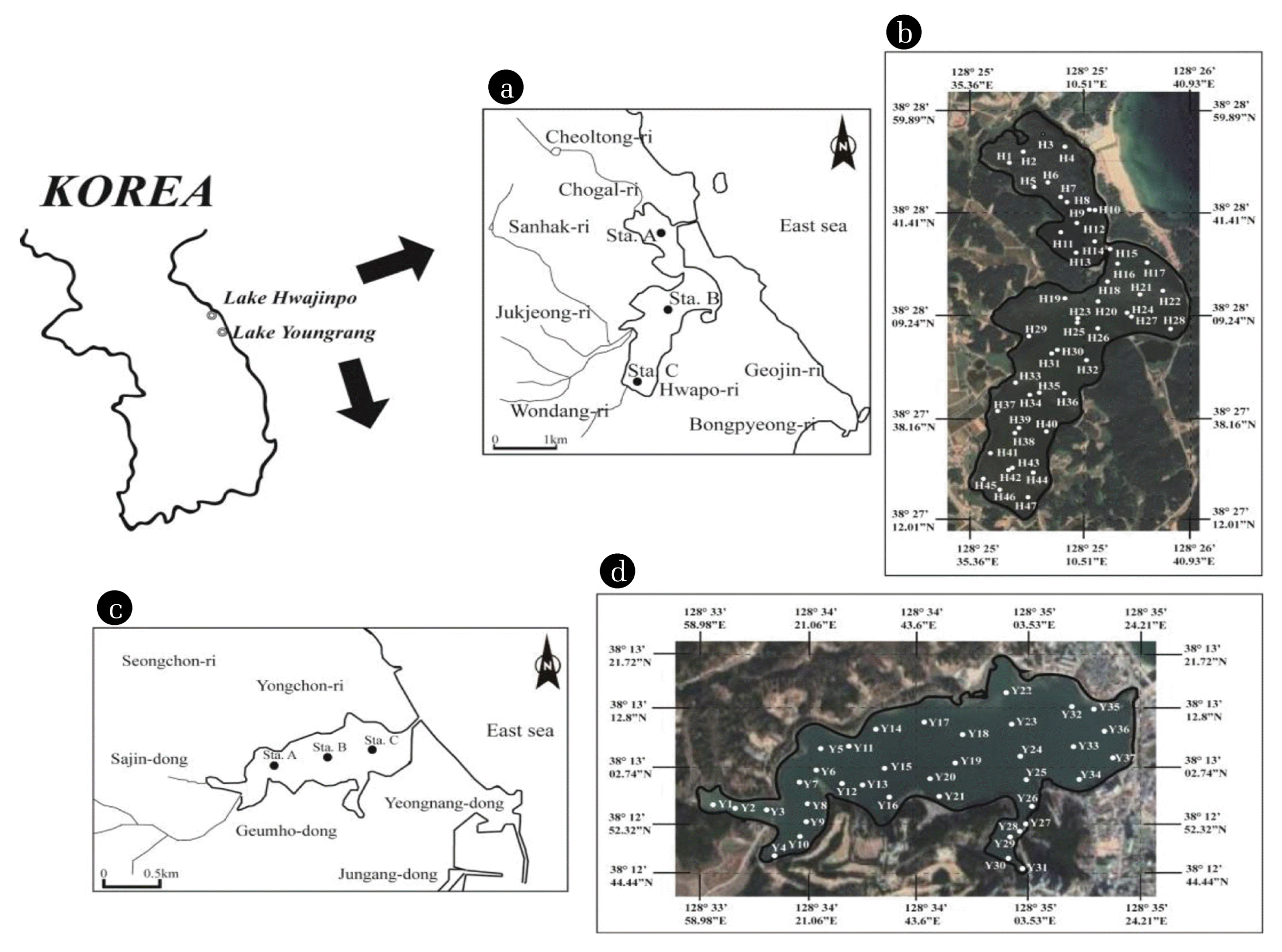
Fig. 3Ternar diagram plots (Shepard 1954) based on sand, silt, and clay ratios according to the vertical (a) and horizontal distribution (b) of bottom sediments in Lakes Youngrang and Hwajinpo. 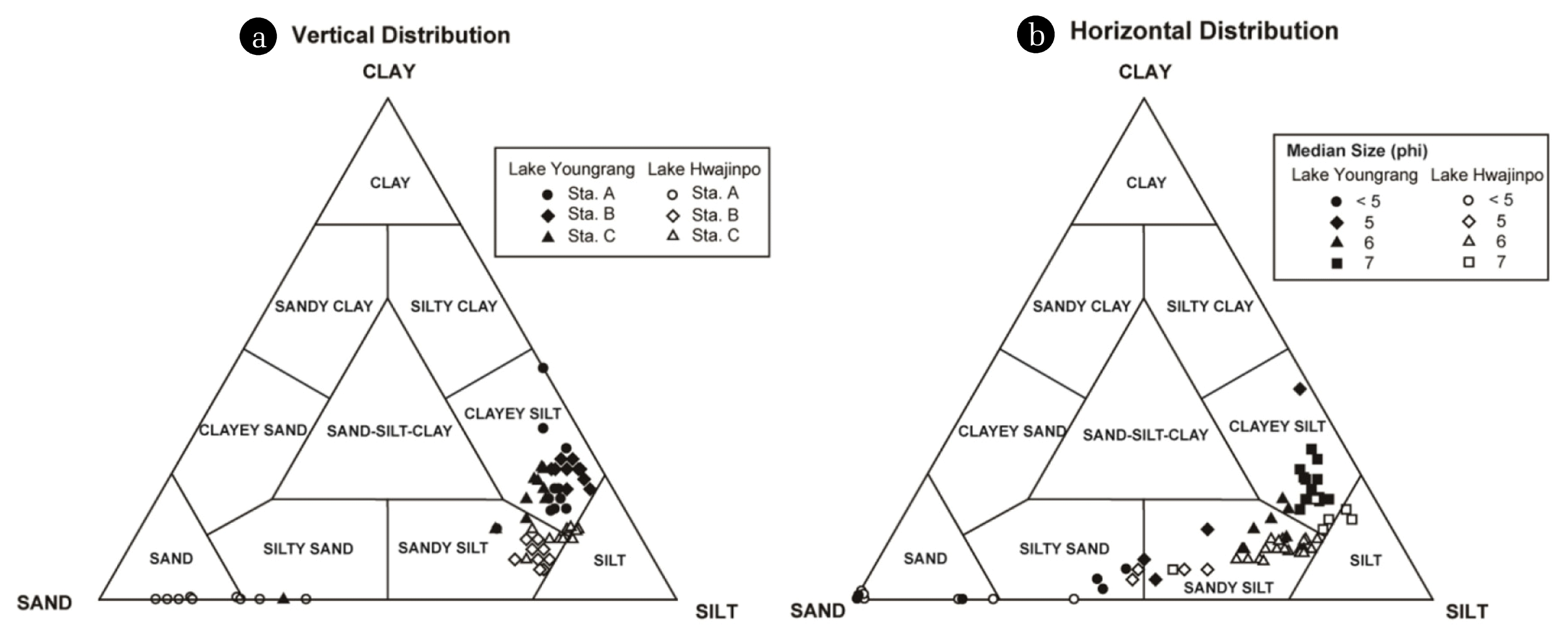
Fig. 4Grain size distribution of bottom sediments in Lake Youngrang (a); Upper panel shows grain size distribution, and lower panel shows a negative correlation between grain size and water depth. Grain size distribution of bottom sediments in the north and south basin of Lake Hwajinpo (b). The left panel shows grain size distribution, and the upper right and lower right panels show a negative correlation between grain size and water depth in the north and south basin of Lake Hwajinpo. 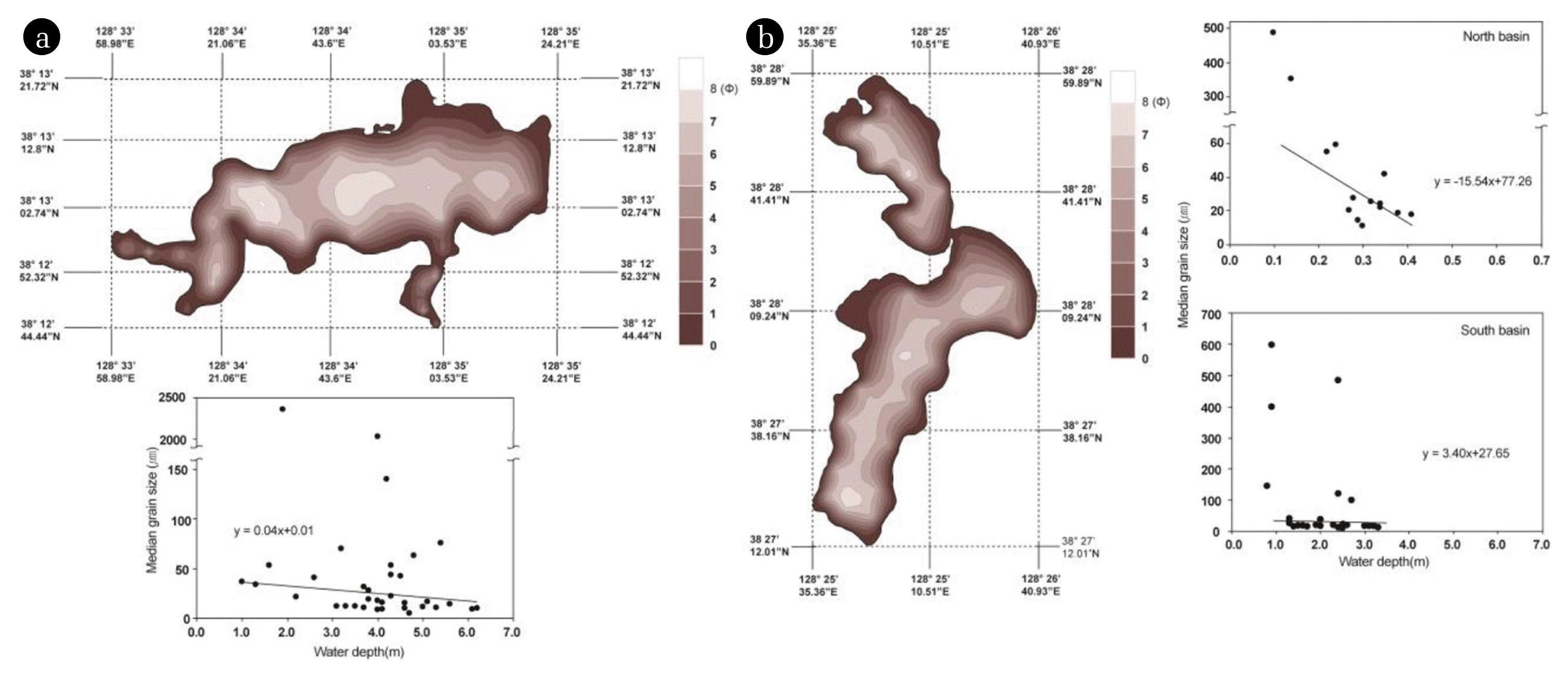
Fig. 5C/N ratio distribution of bottom sediment in Lakes Youngrang (a) and Hwajinpo (b). (a) The upper panel shows the C/N ratio distribution, and the lower left panel shows a positive correlation between carbon and nitrogen contents, whereas C/N ratios have a negative correlation. (a) The left panel shows C/N ratio distribution, the right upper and middle panels show a positive correlation between carbon and nitrogen contents in the north and south basins, and the right lower panel shows positive correlation between the C/N ratios of both basins. 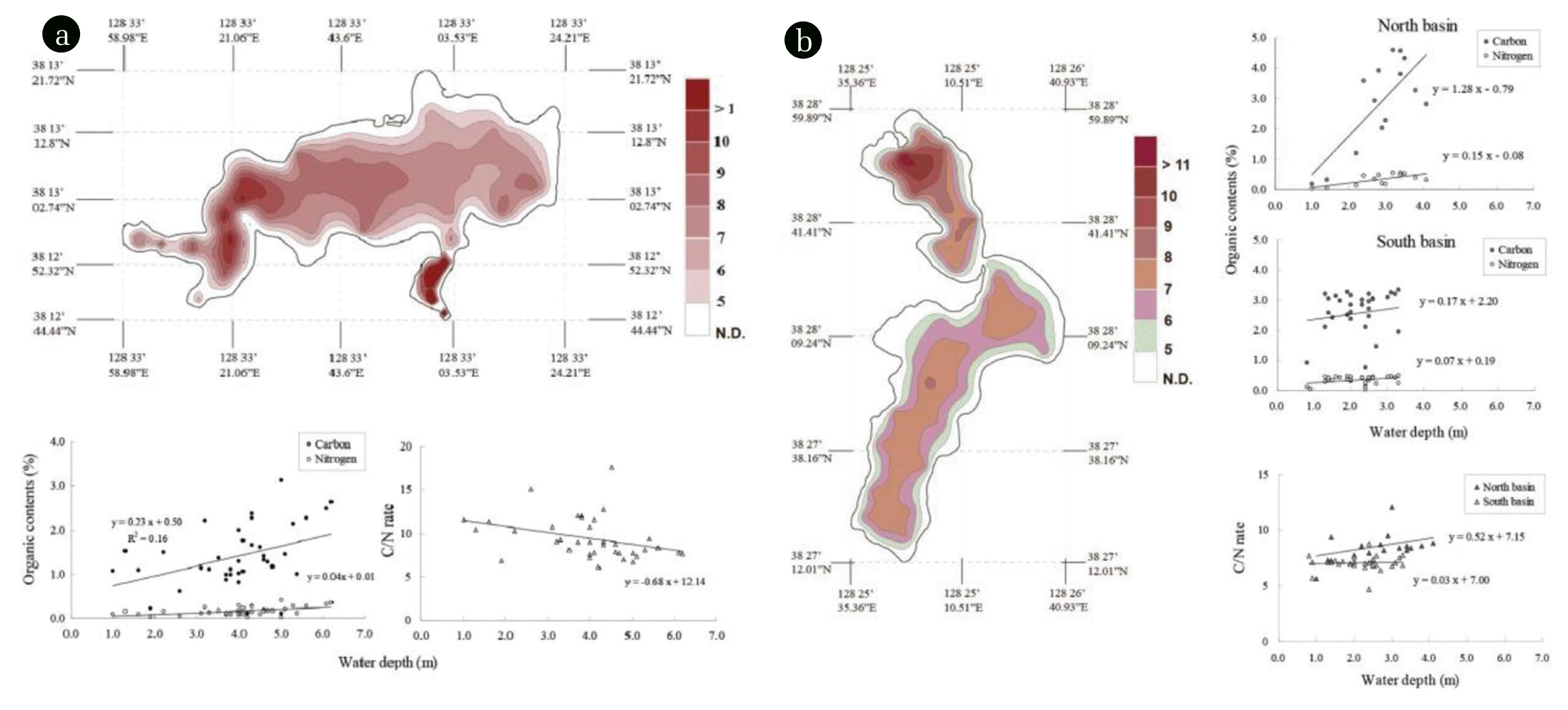
Fig. 6Carbon and nitrogen stable isotope plots (a) for respective stations of both lakes. Black square indicates Lake Youngrang, and gray circles indicate Lake Hwajinpo. Line bar indicates the mean carbon and nitrogen isotope values in each lake. Principal components analysis (PCA; b) of the environmental data, scaling type 2. The first axis explains 46.7% of the variation and the second axis explains 23.4%. Environmental variables: arrows indicate δ15N, δ13C, C/N, and water depth, respectively. “Y” indicates stations in Lake Youngrang, and “H” indicates stations in Lake Hwajinpo. 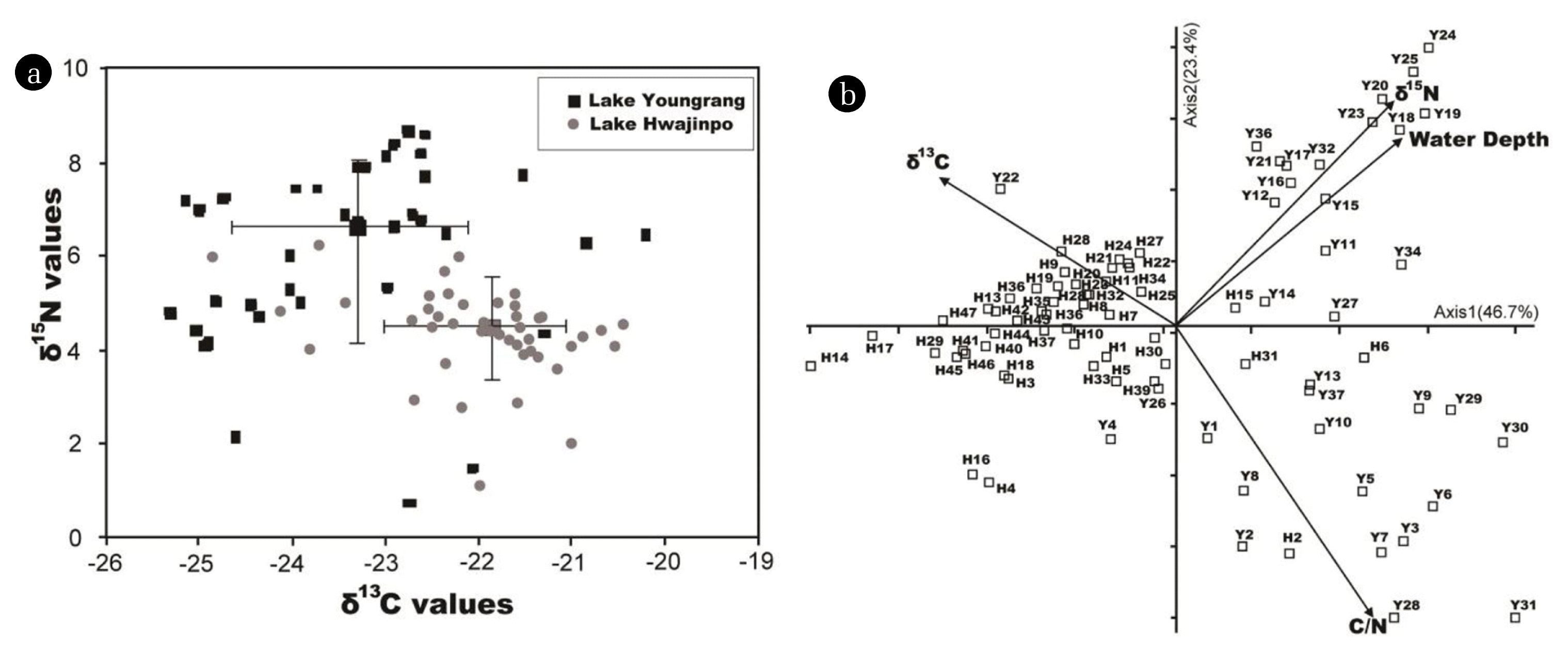
Table 1Vertical Distribution of Bottom Sediment Grain Size (A), total organic carbon (B), total organic nitrogen (C), and C/N ratio (D) in Lake Youngrang Table 2Vertical Distribution of Bottom Sediment Grain Size (A), Total Organic Carbon (B), Total Organic Nitrogen (C), and C/N Ratio (D) in Lake Hwajinpo |
|
||||||||||||||||||||||||||||||||||