1. Introduction
With increasing population growth, there has been a lot of progress in raising the productivity of agricultural production. Modern agriculture should protect its products from the risk of destruction by insects and worms. Hence, pesticides are widely used in the world to safe guard plants from the pests [1]. Due to the high consumption of pesticides, considerable amounts of the pesticides enter the environment (water resources and soil), consequently they have harmful effects on the humans and animals [2, 3]. Organophosphate pesticide is one of an important category of insecticides that are mostly used in agriculture. Chlorpyrifos (Fig. 1) is an insecticide which is widely used in agricultural for the struggle against pests [4]. The acute oral and dermal LD50 for chlorpyrifos in male and female rats are 270 and 2000, respectively [5, 6]. Chlorpyrifos is absorbed through the skin and causing chronic and acute poisoning [7]. The acetyl cholinesterase is an enzyme that hydrolyzes the acetylcholine to stimulate the nerves at the synapse site, the presence of such poisons causes to interact with this enzyme and consequently leads to neurological disorders [8]. As a result, it is essential to be removed the chlorpyrifos in water resources. A number of various methods, such as Fenton oxidation [9], biotreatment [10], have been reported to remove organic chemicals including pesticides. Batch processing and continuous flow absorption methods have been widely considered by employing various natural and synthetic adsorbents as high-performance processes for purification of aqueous solutions. MOFS as porous material, which have the fastest growing knowledge amongst new organic materials, this research area is based on the combination of organic and inorganic branches. These compounds have high porosity, with large specific area. Therefore they are good material to be used them as an adsorbent [11–14].
ZIFS are a set of MOFS materials with chemical and thermal stabilities that are topologically isomorphic with zeolite and contain functionalized organic linked and transition metal ions and their properties have been studied at the presence of these special metals [15–18]. Synthesis of ZIFs with two metals causes to improve their properties, for instance, their porosity and surface area increases compared to ZIF-8 [15]. In this work, the removal efficiency of chlorpyrifos was examined by both of doula metal and single metal ZIF sunder similar circumstances. The parameters of the dose of absorbent, pH and contact time can potentially affecting on the removal efficiency. In this respect, there are several methods including central composite design, Doehlert design and Box-Behnken design (BBD). BBD is an experimental design for identifying a response surface that requires less number of experiments and it is more practical and feasible than other design therefore in this research to find the optimized condition the BBD method was performed [19–21].
2. Experimental Section
2.1. Synthesis ZIF-8 and Dual Metal ZIF
Commercial CMC, ZIFs were synthesized with two metal ions of Co2+ and Zn2+, the prepared samples were characterized with several techniques such as FTIR, XRD, BET and FESEM.
To synthesis ZIF-8,8gr of 2-metylimidasole was added in 25 mL methanol (solution A) and stirred for 7 min; in another vessel 3 gr of Zn(NO3)2. 6H2O was dissolved in 25 mL of methanol (solution B). Solutions A and, B were mixed and stirred for 6 h, then slurry was centrifuged (7,000 rpm) and white the precipitate was washed with methanol for several times and dried at 60°C for 24 h. To synthesis ZIF-2M in the first vessel, 8 g of 2-metylimidasole was added in 25 mL methanol (solution C) and stirred for 7 min; in the second vessel 1.5 g of Zn(NO3)2. 6H2O was dissolved in 12.5 mL of deionized water (solution D); in the another vessel 1.5 g of Co(NO3)2. 6H2O was dissolved in 12.5 mL of deionized water (solution E). Three solutions (A, B & C) were mixed and stirred for 7 h, then slurry was centrifuging (7,500 rpm) and the precipitate (light Purple) was washed with deionized water and dried at 60°C for 24 h.
2.2. Instrument and Software
XRD patterns of ZIF and ZIF-2M was obtained by X-ray diffractometer (PHILIPS- PW1730) using Cu Ka radiation (1.5 Å) in the range of 2q 5–80.
The infrared spectra of ZIF-2M and ZIF-8 were measured by Fourier infrared spectroscopy (AVATAR spectrophotometer) over the range 400–4000 cm−1 at room temperature. The surface morphology and elemental composition of ZIF-2M and ZIF-8 were acquired by field emission scanning electron microscopy coupled with energy dispersive X-ray spectrometer (FESEM/EDX) model TESCAN-MIRA3.
The specific surface area and porosity structure was studied by nitrogen adsorption/desorption isotherms at 77K with BET (BELSORP MINI2) method. UV-vis spectra were recorded by UV-visible spectrometer PG instruments Ltd.
To investigate the effect of different factors on the absorption of cefixime on ZIF-8 and ZIF-2m the BBD method was performed (Design Expert software, version 7, State-Ease, Inc., United States).
2.3. Adsorption Isotherm of Chlorpyrifos Adsorption
Langmuir isotherm (Eq. (1)) and Freundlich isotherm (Eq. (2)) were selected for analyzing adsorption isotherm. Langmuir isotherm is valid for monolayer adsorption on a surface owning to fit identical sites while Freundlich isotherm assumes that the adsorption of molecule occurs on a heterogeneous surface by multilayer adsorption.
Where qe is the adsorbent capacity at equilibrium (mg/g); Ce is the equilibrium concentration of chlorpyrifos (mg/L); qm and KL are maximum adsorption capacity and Langmuir constant.
where Kf and n are Freundlich equilibrium constants and adsorption intensity, respectively [23].
3. Results and Discussion
3.1. Characterization
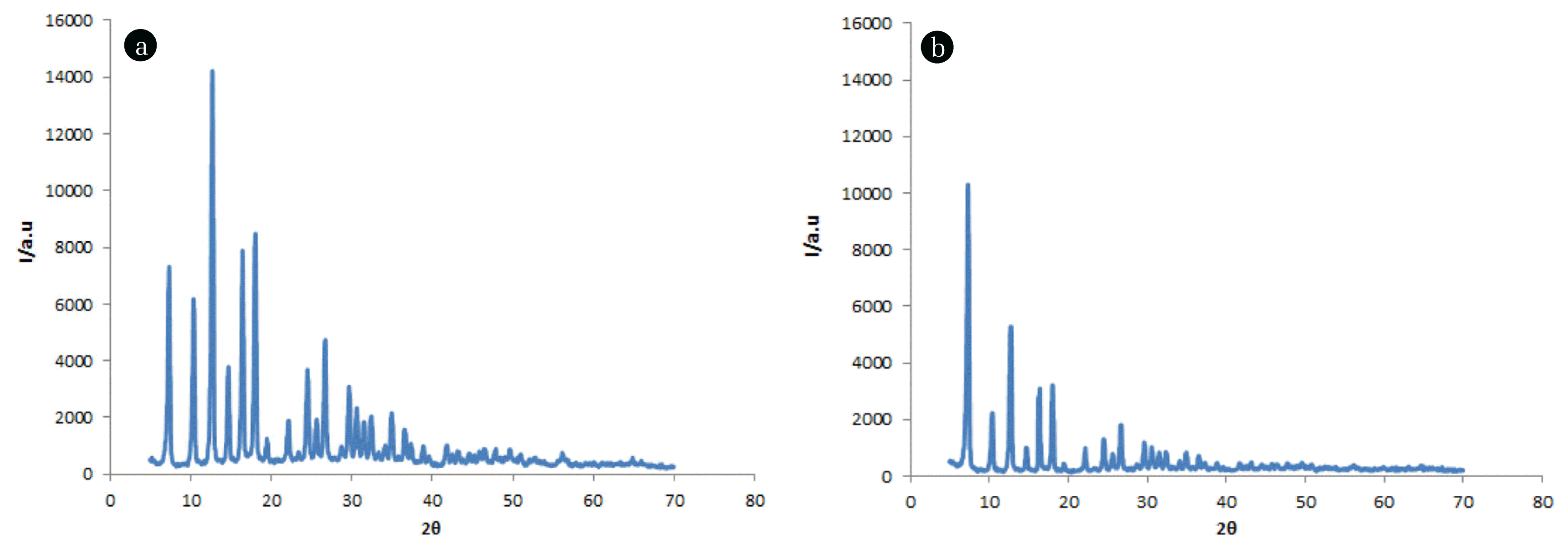
XRD patterns of ZIF-2M and ZIF-8 are presented in Fig. 2. The feature of the XRD patterns reveal both synthesized materials were well crystalized. The crystallinity of the compounds can be estimated by considering the index peak (intense peak) of the XRD pattern. As can be seen the index peak of ZIF-2M (12.7 2 ϴ) has higher intensity than that of ZIF-8(7.3 2 ϴ). As a result, it can be deduced that the ZIF-2M (Fig. 2(a)) was more crystalized than that of ZIF-8 (Fig. 2(b)).
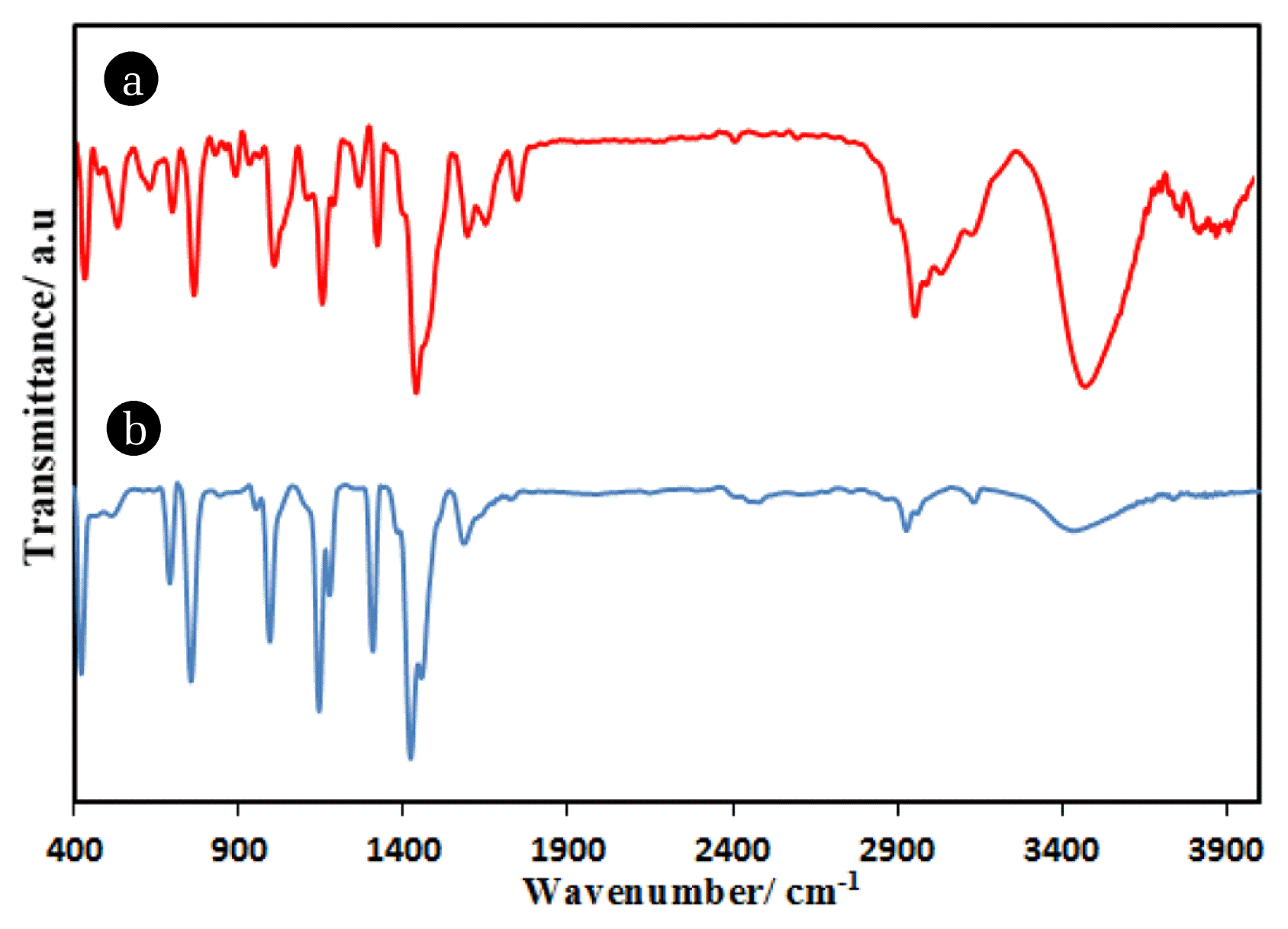
FTIR spectra of ZIF-2M and ZIF-8 were recorded under the same spectral conditions in the range 400–4000 cm−1 and presented in Fig. 3. The peaks at 2925.6 cm−1 and 1576.87 cm−1 are related to stretching mode of C = C and C = N in 2-methylimidazole ring. Also, the bands from the range of 650 to 1500 cm−1 assign to the vibrational modes of the ring for both ZIFs, the peaks at 421 cm−1 ascribed to Zn-N stretching mode vibration and the peak at 490cm−1 for ZIF-2M indicates Co-N bond, confirming bonding between the metals and the organic ligand. Results are in good agreement with previous reports [24, 25].
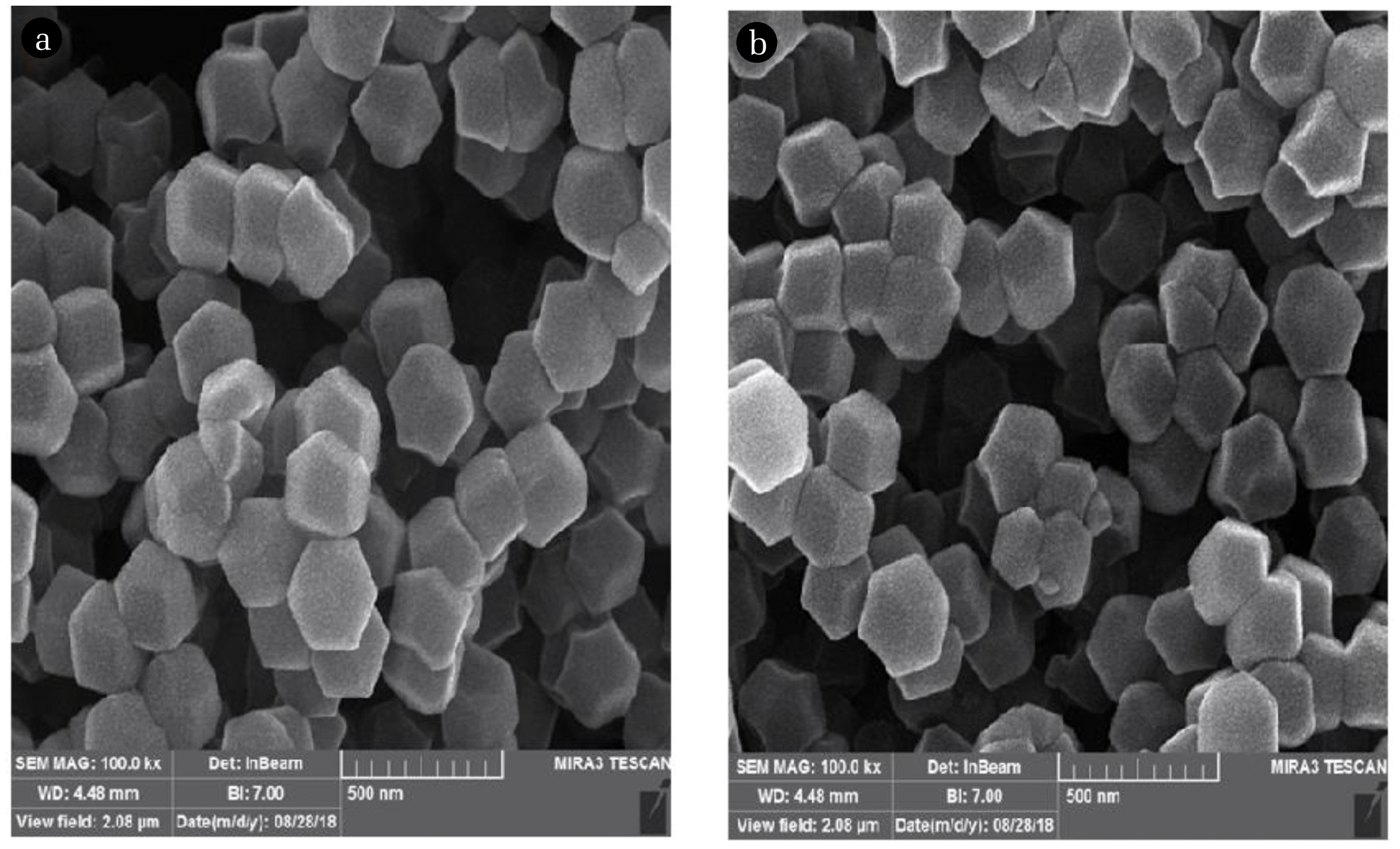
The surface morphology and elemental analysis of ZIF-2M and ZIF-8 were also acquired by the FESEM with 1ˊ105 magnification (Fig. 4). As can be observed the synthesized nanoparticles are fairly uniform with polyhedral shape.
The elemental analysis for both nanoparticles was implemented by EDX and presented in Fig. S1(a) & (b) with corresponding elemental analysis (inserted Table). Fig. S1(a) clearly shows signals corresponding to Zn and Co elements confirming successful synthesis of ZIF-2M. Likewise Fig. S2(a) illustrates the EDX spectrum of ZIF-8 showing signal of Zn element.
Adsorption/desorption isotherms of ZIF-8 and ZIF-2M were performed by BET method and presented in Fig. 5. On the basis of IUPAC classification, the isotherm of adsorption is associated to the type I. The morphological parameters of the corresponding nanoparticles are listed in Table 1. As can be realized two nanoparticles have somewhat difference. The specific area of ZIF-2M is more than that of ZIF-8, also the total pore of ZIF-2M is higher than that of ZIF-8. The mean pore diameter of ZIF-2M and ZIF-8 are 2.615 and 1.982 nm, respectively. As a consequence, it can be said that the ZIF-8 is a micro porous while the ZIF-2M is somehow mesoporous material.
3.2. BBD and Data Analysis
When multiple factors affect the response(here removal efficiency), BBD, which enables us to measure the impact of each of the factors, as well as the impact of interactions between the factors on the response and finding the optimized parameters [26, 27]. The objectives of this study were: (1) to optimize the numerous factors including absorbent dose, pH and contact time for chlorpyrifos removal by ZIF-2M and ZIF-8 and obtain maximum response by applying BBD under RSM; and (2) to verify the fitted models and determine the optimum operational conditions for removal efficiency and comparison of the absorbance of the toxin by each nanoparticle in optimal conditions. For each factor, 3 levels of 0, −1, +1 were selected which are listed in Table 2. Number of experiments was calculated by equation (3). On the basis of this equation, 16 experiments are needed which 12 runs are related to factorial experiments and 4 of them are belong to the central point. Results (removal efficiency) of series experiment are illustrated in Table S2. All experiments were performed with the similar circumstances using 30 ppm chlorpyrifos and removal efficiency was calculated by Eq. (4). To adjust the initial pH of the solutions NaOH 0.1 M and HCl 0.1M were used. At the end of each adsorption process, the solution was centrifuged at 6500 rpm and the supernatant was analyzed with UV-vis spectrophotometer.
Where K is the factor number and CP is the replicate number of the central point [28].
Where, Ci and Ce (mg/L) are the initial and final concentrations of chlorpyrifos, respectively.
3.3. Suitability of the Model
In the BBD to be obtained a suitable model it is necessary to be found the appropriate parameters. These parameters can be estimated by ANOVA results, which were achieved by Design Expert software. The ANOVA results for removal efficiency of chlorpyrifos by using ZIF-2M and ZIF-8 are listed in Table S3 and Table S4, respectively. From Tables S3 and S4, the model F value for sorption chlorpyrifos by ZIF-2M and ZIF-8 were 848.17 and 676.72, respectively. Probability, P value of < 0.0001 for two models indicates that the model is significant. Also for two models The F value less than 0.0001 indicates that the parameter and its interactions with the other parameters is important.
According to above results, it can be deduced that factors of pH and absorbent dosage are very important factors for adsorption of chlorpyrifos by ZIF-8 and ZIF-2M. The coefficient of determination (R2) values for ZIF-2M and ZIF-8 are 0.99. The normal Plot diagrams (Fig. S2) indicates there is a good linear correlation between the experiments and the predicted data. Lack of fit data is essential to check the compatibility of a model for predicting the optimum response. In this case, for two models the F-value for lack of fit is very low and indicating it is not significant relative to the pure error. To detect the effects of uncontrollable variables on the response during the experiments the residual curves were investigated. Corresponding curve for the removal of chlorpyrifos is illustrated in Figure S3. As can be observed, there is no meaningful error in the removal efficiency chlorpyrifos. This graph also suggests that there are no systematic errors in the response to the experiments. As a result, it can be concluded that the predicted models are suitable for optimization parameters in adsorption process of the chlorpyrifos by both synthesized nanoparticles.
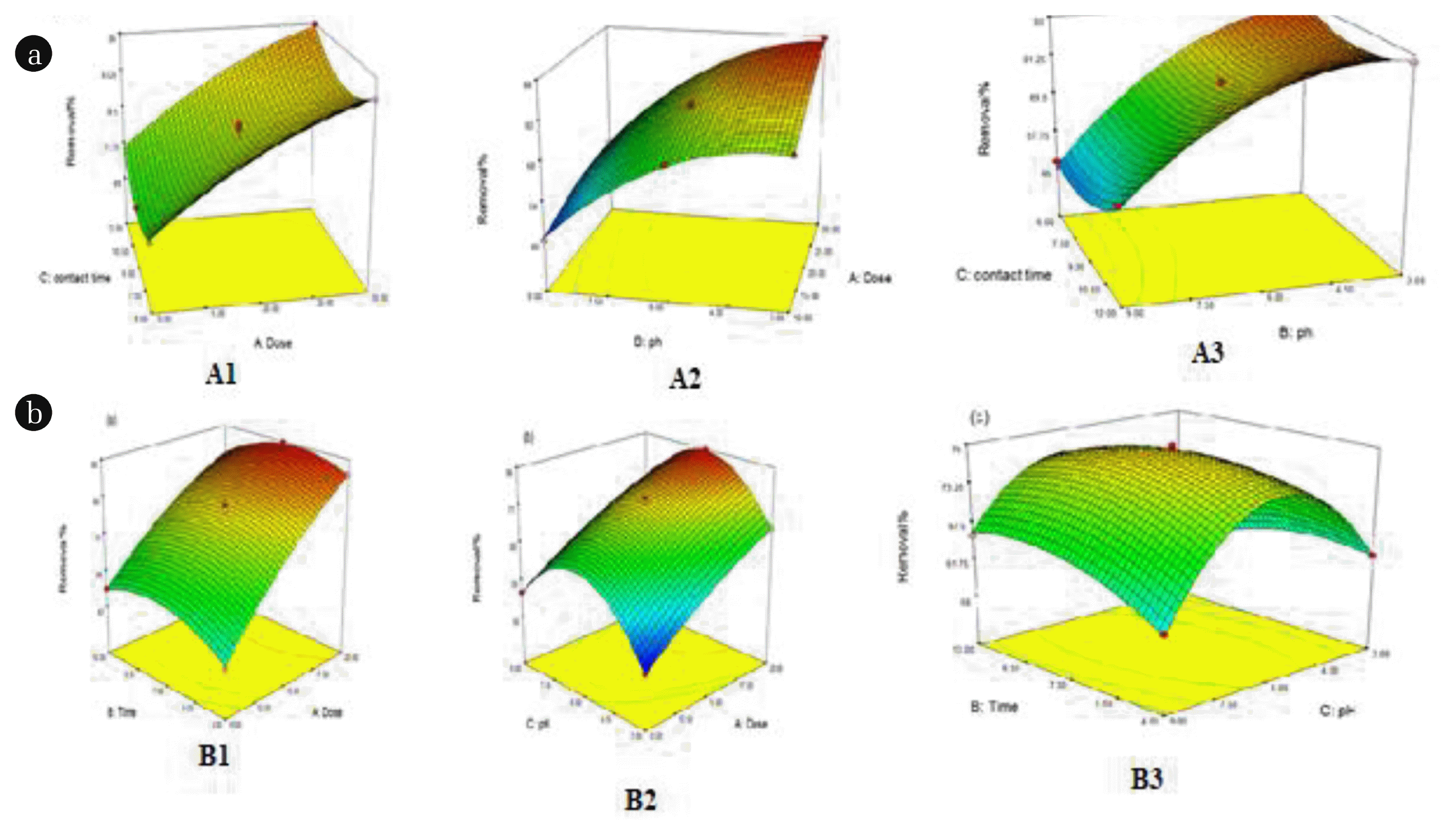
3.4. Response Surface
3-factor and 3-level BBD methods were selected to evaluate the interaction effects of three independent variables including absorbent dosage, pH and contact time on the chlorpyrifos removal efficiency. The relationships between the independent variables and response values can be deduced in 3D response (Fig. 6).
On the basis of the results, it can be concluded that the behavior of sorption chlorpyrifos on both of adsorbent (ZIF-2M and ZIF-8) are similar for all of circumstances. Results indicated that the pH close to 7.0 is an optimum value for this proposes. It can be said that electrostatic interaction is governing between adsorbate (chlorpyrifos) and both adsorbents. The chlorpyrifos molecule as no ionic form that means neither cationic from in the acidic media (pH < 7) and nor in basic media (pH > 7) therefore it can be said that there is no dipole-dipole interaction between the adsorbent (ZIFs) and the adsorbate (chlorpyrifos). It would be mentioned that the zeta potential of both ZIFs are about 9.8 [29]. Here we can say the π-π interaction is dominated between the adsorbent and the adsorbate. This is justified by considering the structure of chlorpyrifos which is an aromatic molecule and the other hand the ZIF-2M and ZIF-8 have the imidazolate molecule in their structures. As a result it can be concluded the π-π interaction is dominated interaction.
Another effective factor is absorbent dosage that was studied at three levels of 10, 15 and 20mgr. As can be observed, for both nanoparticles the removal efficiency increases with increasing dose of absorbent, since, the available sites are raised. Contact time also is another factor that can influence on the response, obviously by increasing contact time the removal percent is increased however, after a given time reach it has no impact. In this experiment, the optimized values of adsorbent dosage, pH and contact time were obtained 17.85 mgr, 6.33 and 7min for ZIF-2M and those for ZIF-8, 19.5 mgr, 6.8 and 10 min
3.5. Study of Removal Efficiency at Optimal Levels
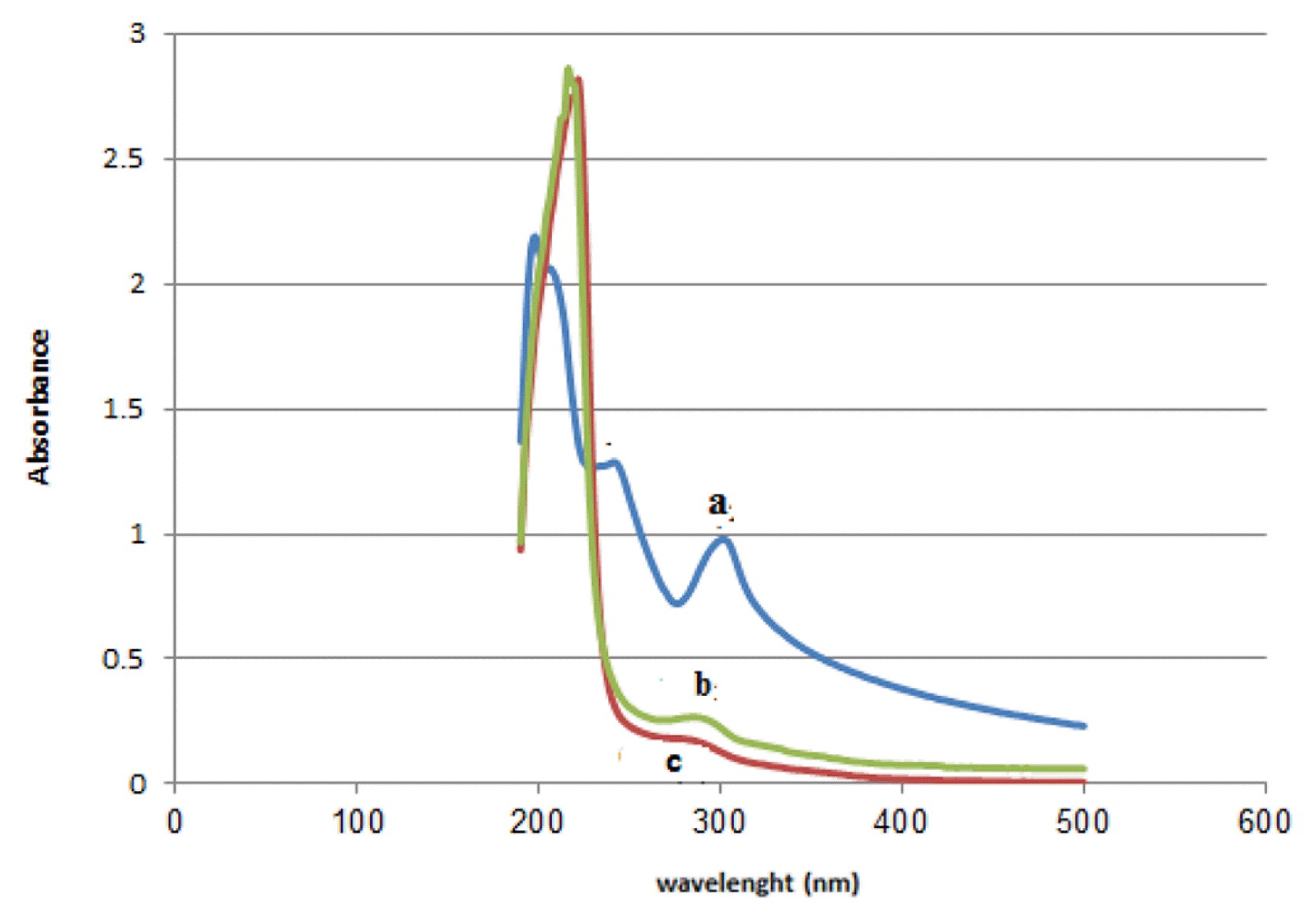
The removal efficiency of chlorpyrifos using two nanoparticles of ZIF-2M and ZIF-8 was evaluated at optimal levels. The experiments were performed under optimal condition as expressed above. Results revealed that ZIF-2M nanoparticles more efficiently remove the chlorpyrifos than that ZIF-8. So that that the removal% for the former was 97% and that for the latter was found 90%. It would be pointed out that the dosage of ZIF-2M is less than that ZIF-8 in similar circumstances. The UV-vis spectra of corresponding results are shown in Fig. 7.
3.6. Adsorption Isotherm
According to the above results, series experiments were performed at optimal condition (pH = 6.33, absorbent dose = 17.85 mg, contact time = 7 min) with different initial chlorpyrifos concentrations. The plot of Ce/qe verses Ce (Langmuir isotherm) and log qe against log Ce (Freundlich) were constructed by extracting data adsorption. The values of parameters of qmax, KL, Kf, and n were calculated and listed in Table S4. By comparison and considering of data extracted from the plot of Langmuir and Freundlich models (Fig. S4(a) & (b)), it can be deduced that the former model is more suitable model than that the latter. Since the determination coefficient for Langmuir (R2 = 0. 988) has higher value than that Freundlich model. This explains that the adsorption process is dominated by single layer adsorption.
4. Conclusions
Free solvent synthesis of dual metal ZIF (ZIF-2M) nanoparticle was performed containing Zn and Co metals. ZIF-2M was used to examine for removal of chlorpyrifos as an organophosphate poisoning. Experiment conditions were optimized assessing BBD. Removal efficiency of chlorpyrifos with ZIF-2M and ZIF-8 were examined and results revealed that the ZIF-2M has more removal efficiency than that of ZIF-8. Also the removal of chlorpyrifos can be taken place in neutral medium (pH » 6.5). Studies of isotherm models indicated that the adsorption process is preferably occurred by single layer adsorption (Langmuir model). As a conclusion, dual metal ZIF is good adsorbent with improved chemical and physical properties with keeping the basic structure of ZIFs.