AbstractWeirs are conventional structures that control water level and velocity in streams to facilitate water resource management. Despite many weirs built in streams, there is little information how weirs change hydrology regime and how that translates to sediment and phosphorus (P) responses. This study evaluated the influence of weirs on P retention and mobilization in an urban tributary of the Han River in Korea. Total P concentrations in sediments upstream of weirs were higher than the downstream site, mainly due to the increase of potentially available fractions (labile P and aluminum- and iron-bound P) (p < 0.05). Equilibrium phosphorus concentrations (EPCo) were lower than soluble reactive phosphorus (SRP) concentrations of stream waters, but there was an increasing trend of sediment EPCo upstream of weirs compared to the downstream site (p < 0.001) indicating a greater potential for P release upstream of weirs. Sediment core incubation showed that SRP release rates upstream of weirs were higher than the downstream site under anoxic conditions of the water column (p < 0.01), but not under oxic conditions. SRP release rates under anoxic conditions were greater than that measured under oxic conditions. Un-neutral pH and increased temperature could also enhance SRP release rates upstream of weirs. We conclude that weirs can increase P retention within stream sediments and potentially promote significant P releases into waters, which in turn cause eutrophication.
1. IntroductionUrbanization has led to an increase in phosphorus (P) loading into streams [1]. Typically, high percentage of the P loads is trapped and processed upstream of impounding structures in streams [2, 3]. The impoundment of streams, even with low-head structures, such as weirs, alters hydrology by slowing flow velocities (or increasing hydraulic retention time and hydroperiod), thereby affects sediment dynamics and P cycling [2, 3]. Such excessive P loading under the control of stream flows regulated by impounding structures is of primary concern as this can cause the seasonal eutrophication of streams and socioeconomic problems at regional scales [4–6].
In urban streams, weirs are usually installed for flood control as flooding can be exacerbated by increased amounts of impervious surface [7, 8]. As such, weirs have created stepped stream systems where the hydraulic energy is highly reduced by the reduction of the slope and allow more time for dissolved P to interact with sediments and for particle-bound P to settle out [9–11]. Since the early 1980s, sediment has been identified as the primary pollutant of concern for stream waters. To address this concern, many management practices have been promoted to enhance nutrient management in the United States [12]. Recently, the installation of weirs in agricultural drainage ditches is being advocated as an innovative strategy to improve water quality of downstream ecosystems in the Lower Mississippi Fluvial Valley, USA [13]. The intention of weir installation in agricultural drainage ditches is to reduce sediments and nutrients like P from entering aquatic systems (rivers and streams) by altering ditch hydrology [10]. Although promoting sedimentation and P retention through increased retention time is the premise under which weirs are installed, there are several uncertainties about the way weirs change hydrology regime and how that translates to P and sediment responses in aquatic systems [11, 14, 15]. Information in details regarding influences of weirs on sediment retention and the associated P dynamics is still scarce [13].
Large proportions of P sources into urban streams are in a soluble form as mainly derived from municipal wastewater treatment plants [16]. Highly bioavailable dissolved P may be converted into less-bioavailable particulate and organic fractions as the consequence of uptake onto sediments or by phytoplankton under low flow conditions [2]. Dissolved P retention by sediments, together with particulate P deposition, during low flows permanently contributes to large stores of P in sediments upstream of weirs, especially in streams with high point source P loads [11, 13]. A portion of these P pools will be flushed down the stream during subsequent high flows. However, some previous studies have particularly shown the lack of net remobilization of the P pool associated with sediments above the weirs under highest flows in urban streams [11, 14]. Sediments above the weirs of urban streams thus potentially constitute long-term sinks, and consequently long-term sources, of P for years, decades and even centuries [17]. This P pool has been referred to as legacy P, which is particularly problematic to the watershed conservation management [15, 18]. The spatial-temporal variations of hydraulics, hydrology, morphology and land management clearly exert interacting controls on the interaction between sediments and stream water and the lag between P storage and release [15]. Quantification of phosphorus uptake/release mechanisms along with the sedimentation processes above the weirs would be of value to better understand P dynamics and further help to control eutrophication.
Research suggests that weirs can induce not only hydrological changes but also physicochemical changes of stream water including temperature, dissolved oxygen and pH [19]. Changes in stream water chemistry in turn can affect sediment conditions; for example, by controlling the diffusion of dissolved oxygen into sediments, by determining oxygen demands for microbiological activities, and the protonation state of iron and aluminum oxide and hydroxides [20–22]. Sediments are especially considered as long-term sources of P in aquatic systems after the external P inputs from the watershed have been reduced [5, 15]. The magnitude of the phosphorus flux across sediment-water interfaces is substantially variable under effects of many environmental factors, for example ranged from 2% to 28% of the retained P in stream sediments in the basin of Lake Okeechobee [23]. Generally, sediment phosphorus fluxes are attributed to changes in redox conditions at the sediment-water interface [24]. The limited P efflux of oxic sediments is due to the strong adsorption of dissolved P to solid iron oxyhydroxides, whereas under anoxic condition, iron oxyhydroxides reductively dissolve and release phosphorus into the water column [25, 26]. Over the last decades, a number of alternative P release controls have been suggested, including the sediment composition (ratio of FeOOH and Al(OH)3), the decomposition of organic phosphorus and the regulation of bacterial phosphorus as well as pH condition [20, 27, 28]. Sulfate-controlled P release can also explain several apparent deviations from the classical paradigm in sulfate-rich sediments [29]. However, effects of sulfate on sediment P releases in freshwater systems are small compared to that in saline systems with high sulfate concentration [30]. The indirect influences of weirs on the fate of sediment P pools via controlling water chemistry may finally result in a positive feedback toward greater availability of P in streams.
This study tested the hypothesis that weirs significantly increase the amount of P accumulating in sediments of an urban stream associated with point sources and examined the effects of weirs on the potential mobility of P retained. We aim 1) to describe the variations in patterns of different P fractions in streambed sediments across weirs, 2) to describe the dynamics of P in streambed sediments deposited upstream of weirs, and 3) to discuss the environmental factors mediating the direction and magnitude of phosphorus fluxes between sediments and water body upstream of weirs.
2. Materials and Methods2.1. Study Site and SamplingThe Tanchun Stream in South Korea was surveyed to examine the trends of P release from sediments upstream of weirs. Tanchun Stream is one of contaminated tributaries in the basin of the Han River, the largest river in South Korea located near the center of the Korean peninsula. Sediment and water sampling was conducted in a 2-km stretch of Tanchun Stream downstream from Tuncheon Bridge (37° 25′ 41″ N; 127° 07′ 02″ E) (Fig. 1). This section has a high degree of impact from several sewage treatment works and its flow is controlled by two free overfall weirs, spaced approximately 100 m apart. The weirs have a relatively broad crest (1 m × 1 m; width × height) and made up of cement-based materials across the whole width of the channel (48–55 m). Further details of the weir construction are unfortunately unknown. The Tanchun Treatment Plant is a combined sewage treatment plant among four sewage treatment plants in Seoul city, serving a population of 1,720 × 103. The Tanchun Treatment Plant has the capacity to treat up to 890 × 103 m3 of sewage water per day [31].
Samples were collected in May 2012 at two locations, about 50 m upstream of the first weir (W1) and downstream of the second weir (W2) (Fig. 1). During the sampling time, the mean discharge was about 7.5 m3/s. The water level was extremely low. In the middle of the stream, the water depth was about 1 m upstream of the weirs and decreased to half a meter downstream of the weirs. No discharge was observed from the sewage treatment plant during the sampling time. The sediments are loamy sandy upstream and sandy downstream of the weirs at the surface (0–10 cm) and are underlain with gravel.
Triplicate intact sediment cores (10 cm i.d.), consisting of the top 10 cm of streambed sediments, were collected at each sampling location for the P flux measurement. Other triplicate sediment cores (5 cm i.d.) were also collected at each sampling location for biological and chemical analysis. For the kinetics experiments, eighteen intact sediment cores (10 cm i.d.) and stream water (30 L) were sampled at one location upstream of the weirs, chosen randomly and different from the sampling locations to examine the interacting effects of water pH, dissolved oxygen (DO) and temperature to the P release from streambed sediments.
Triplicate water samples (2 L) were also taken at each station for the analysis of total suspended solid (TSS), soluble reactive phosphorus (SRP), and anion concentration (NO3− and SO42−). All samples were transported to the laboratory immediately within 6 hr after being taken. Water and sediment samples for biological and chemical analysis were transported at 4°C using ice boxes.
2.2. Biological and Physicochemical MeasurementsOn-site measurement of pH, dissolved oxygen, temperature, and redox potential were made using portable meters. Each sediment core (5 cm i.d.) was homogenized before the analysis of concentrations of inorganic phosphorus fractions. The homogenized sediment samples were also subjected to other physicochemical measurements, such as bulk density (Db), pH, water content, organic matter content (SOM), total phosphorus (TP) concentration, cation exchange capacity (CEC), total Ca, Al, Fe, and Mg concentration, extracellular enzyme activities (β-glucosidase, N-acetylglucosaminidase, phosphatase, and arylsulfatase), and equilibrium phosphorus concentration (EPCo).
The TSS (mg/L) was determined by dividing the oven dry weight of the solid retained onto a 0.45-μm filter, after filtering a portion of the river water, by the volume of water [30]. Nitrate (NO3−) and sulfate (SO42−) concentrations were analyzed using ion chromatography (Dionex, Sunnyvale, CA, USA) after filtering through a 0.20-μm filter. SRP concentration of river water was determined using the ascorbic acid method [32].
Dry bulk density (kg/L) was determined by dividing the oven dry weight of the sediment by its bulk volume. Sediment pH was determined in a solution of 1:2.5 sediment:water using a glass electrode (Orion 3-Star Plus Benchtop pH; Thermo Scientific, Waltham, MA, USA). SOM (% of dry sediment) was measured based on the weight loss in 2.5 g of sediment burned at 550°C for 24 hr, following oven drying at 105°C for 24 hr for determination of water content [33]. Sediment extracellular enzyme activities (β-glucosidase, N-acetylglucosaminidase, phosphatase, and arylsulfatase; nmol/g/min) were measured according to Kang et al. [34]. The CEC was determined by colorimetric method according to Aran et al. [35]. The 2.5 g of wet sediment was shaken with 40 mL cobaltihexamine chloride (0.05 N) in a 50 mL polypropylene tube for 1 hr on a rotating shaker at 60 rpm, and then centrifuged at 7,000 g for 10 min. The supernatant was filtered through a 0.22-μm filter and the absorbance was measured at 472 nm. The CECA472 (meq/100 g or cmol+/kg) was calculated as:
where A4720.05N and A472assay are the absorbencies at 472 nm of 0.05 N (50 meq/L) cobaltihexamine chloride solution and of the sample supernatant; V is the volume in L of 0.05 N cobaltihexamine chloride solution added to the sediment sample (0.04 L); and m is the sediment dry mass (2 g).
Metal extraction was conducted using aqua regia solution (3 HCl:HNO3). A portion of sediment was air-dried, passed through a 2-mm sieve, and ground using a grinding machine. The 3 g of dried sediment was boiled with a mixture of 21 mL HCl (12 M) and 7 mL HNO3 (15.8 M) for 2 hr. The aliquot after digestion was filtered using ashless filters (Whatman No. 400; Whatman, Maidstone, UK). Total concentrations of metals (Fe, Al, Mg, and Ca) in aqua regia aliquots were determined by ICP-MS [36].
2.3. P Fractionation and Total P MeasurementThe non-calcareous wet sediments collected from Tanchun Stream were subjected to a sequential inorganic P fractionation [37]. The same procedure was applied successfully to fractionate the inorganic P forms in the non-calcareous streambed sediments of Han River in a previous study [38].
To measure total P concentration (g/kg), the aliquot after digestion for metal measurements was diluted with distilled water by a factor of 10. After the dilution, the aliquot was subjected to the measurement of total P concentration using the ascorbic acid method [32].
2.4. Sediment Core Incubation Experiment for the Kinetics of P ReleaseP flux measurements were based on the increase in the SRP concentration of the overlying water when the cores (10 cm i.d.) were incubated oxically and anoxically for 10 to 15 days in the dark in plant growth chambers (VS-3DMC; Vision Scientific, Daejeon, Korea). The water volumes collected together with sediment cores were discharged and refilled with filtered river water immediately before the incubation. Care was taken to minimize any disturbance to the sediment cores. Each core contained a volume of 0.785 L of river water (ca. 10 cm depth). The temperature and pH of the water in each sediment core was controlled during the incubation to reflect the in situ conditions at the sampling time. In doing so, the water in each sediment core was bubbled with gas mixtures during two successional stages. Firstly, the water was bubbled with air/CO2 mixture for 10 days at a position of 5 cm above the sediment-water interface. Secondly, the water was exchanged with new water and continuously bubbled with N2/CO2 for a further 10 days. The bubbles provided the mixing necessary to eliminate concentration gradients in the water column without physically disturbing the sediment-water interface. We used HCl (1 M) and NaOH (1 M), in addition to the adjustment of CO2 bubble flows to control pH levels approximating the expected conditions. Water pH and DO were measured simultaneously using portable pH and DO meters inserted through a hole in the top cap of the core, which was stoppered during the experiment and only opened when the monitoring was needed.
The initial SRP concentrations of the overlying water were determined after 5 hr of gas bubbling. A 4-mL aliquot of overlying water was removed twice a day for the first three days of the incubation and daily over the remaining incubation period using syringes via tubing inserted into the water column. The SRP concentration of the water removed was immediately determined using the ascorbic acid method after being filtered through a 0.45-μm filter [32]. The cumulative release rates of P (mg/day) were determined through a least square linear regression of the mass of P in the overlying water over time. A calculating correction was added to account for the phosphorus removed at each interval measurement [39]. Areal release rates (mg/m2/day) were calculated as the cumulative release rate divided by the surface area of the sediment in the core (m2). The calculation was based on the initial linear portion of two-phase curves of P releases and reported in this study as maximum release rates.
Kinetic experiments measuring the SRP release rates in sediment cores (10 cm i.d.) were also conducted similarly to investigate the interacting effects of temperature (15°C and 25°C), water oxic/anoxic condition (DO > 7.0 mg/L and DO < 0.5 mg/L), and water pH (pH 3.0, 7.0, and 11.0).
2.5. Equilibrium Phosphorus ConcentrationEPCo was determined from batch equilibrium experiments [40]. A 0.75-g air dried sediments were equilibrated for 24 hr in 25 mL CaCl2 solutions (0.01 M) containing different amounts of Ca(H2PO4)2 (0, 0.2, 0.5, 1, and 3 mg/L of P). The change in the amount of SRP adsorbed (Δ[Psed]) after the 24 hr-incubation was plotted against the concentration of SRP in solution ([Pw]f) after the 24 hr-incubation. The Freundlich isotherm was used to fit the data using a least squares method [41]. EPCo (mg/L) is the x-intercept of fitting curves indicating the SRP concentration of the suspension at which no net adsorption or desorption takes place (Δ[Psed] = 0). The y-intercept of the fitting curves indicates the native adsorbed phosphorus (NAP). EPCo is an empirical reference point on the sorption curve, permitting a direct estimate of the capacity of the soil or sediment to adsorb or release phosphorus if the concentration is changed. The linear adsorption coefficient Kd (the buffer intensity at the EPCo) is estimated as the slope of the isotherm curve. Kf is the Freundlich coefficient, and n is the exponential factor (Fig. 2).
2.6. Statistical AnalysisOne-way ANOVAs were run to test the impacts of weirs on water and sediment quality. Repeated-measures ANOVA analysis was used to analyze the differences in the SRP release rates of sediments across weirs. Variations between site (upstream or downstream), incubation condition (oxic or anoxic) and the interaction between incubation and site were analyzed for SRP release experiments. The relationships between variables were evaluated using correlation analysis. PASW Statistics ver. 18.0 software (SPSS Inc., Chicago, IL, USA) was used for all analyses.
3. Results3.1. Water ChemistryNo significant difference was observed between water temperature, NO2− and NO3− concentration over the weirs (Table 1). Water temperature ranged from 25°C to 26°C. The SRP concentrations increased over weirs without a statistical significance (from 0.364 ± 0.011 mg/L upstream to 0.391 ± 0.012 mg/L downstream of weirs). Significant variations over weirs were observed in pH, DO, TSS, and SO42− concentration of the water (Table 1). The most striking difference was seen in the TSS profile across the weirs. TSS concentrations decreased from 24.0 ± 5.0 mg/L upstream to 9.3 ± 2.6 mg/L downstream of weirs. Water had a mean pH of 7.93 ± 0.50 upstream and of 9.19 ± 0.02 downstream of weirs. DO concentrations decreased from 11.4 ± 0.2 mg/L upstream to 8.9 ± 0.3 mg/L downstream of weirs. SO42− concentrations, however, increased from upstream to downstream (Table 1).
3.2. Physicochemical Properties of SedimentsOverall, streambed sediments exhibited distinct characteristics over the weirs (Table 1). Sediments upstream of the weirs contained significantly high TP concentrations (558.131 ± 30.251 mg/kg) and high SOM contents (5.05% ± 0.70%) compared to sediments downstream of the weirs, which contained 203.489 ± 19.487 mg/kg and 1.78% ± 0.10% of TP and SOM, respectively. The CEC of sediments also decreased significantly downstream. In contrast, the redox potential and bulk density of sediments increased significantly downstream in which redox potentials were negative for both upstream and downstream sediments. The total concentrations of metals (Fe, Al, Mg, and Ca) decreased significantly downstream (Fig. 3(a)). In addition, sediments upstream of the weirs exhibited significantly higher activities of β-glucosidase, phosphatase, and sulfatase than sediments downstream of the weirs (Fig. 3(b)). However, no significant differences in pH and temperature were observed between sediments over the weirs. The mean temperature of sediments was from 23.3°C to 25.2°C and the mean pH was from 6.99 to 7.11 (Table 1).
Correlation analysis indicated that TSS negatively correlated with bulk density (r = −0.871, p < 0.5), whereas it positively correlated with SOM (r = 0.923; p < 0.01), TP (r = 0.913; p < 0.05), CEC (r = 0.846; p < 0.05), metal concentrations (r = 0.908, 0.960, 0.907, and 0.880 for Fe, Al, Mg, and Ca, respectively; p < 0.05) and extracellular enzyme activities (r = 0.861 and 0.899 for β-glucosidase and phosphatase, respectively; p < 0.05). In addition, TP significantly correlated with SOM (r = 0.912; p < 0.05), as well as with total Fe (r = 0.966; p < 0.01), Al (r = 0.945; p < 0.01), and Mg concentration (r = 0.953; p < 0.01) in sediments.
3.3. Phosphorus Fractions in SedimentsWe found significantly higher concentrations of NH4Cl-extractable P, NH4F-extractable P, and NaOH-extractable P fractions in upstream sediments compared to downstream sediments of the weirs (Fig. 4). Moreover, NH4Cl-extractable P, NH4F-extractable P, and NaOH-extractable P fractions were significantly positively correlated to TSS (r = 0.954, 0.898, and 0.868, respectively; p < 0.05). NH4Cl-extractable P or labile-P (the most available P fraction in sediments) only accounted for a small portion of the total P in sediments, which was about 4%–5% of the total P (Table 2). Phosphorus adsorbed and precipitated with Fe and Al (non-apatite P or NAIP), which was calculated as a sum of P fractions extracted with NH4F, NaOH, and sodium citrate-dithionite-bicarbonate (CDB), was the dominant portion of the total P of sediments. NAIP represented from 51% to 60% of the total P (Table 2). The second largest P portion contributing to the total P of sediments was phosphorus precipitated with Ca and/or Mg (apatite P or AIP). AIP was extracted with H2SO4 and contributed from 25% to 31% of the total sediment P (Table 2). The remaining un-extractable P fraction (residual-P), which was mainly organic-bound P, accounted for about 10%–11% of the total P of sediments.
3.4. Equilibrium Phosphorus ConcentrationThe Freundlich isotherm fitting of equilibrium adsorption-desorption data enabled the quantitative characterization of sedimentary behaviors as a source (or a sink) of phosphorus to the overlying water. The results showed that the EPCo of sediments was lower than SRP concentrations in water columns (Table 3). We also found significant decreases of EPCo, NAP, and n of sediments from upstream to downstream of the weirs. In contrast, Kf and Kd increased from upstream to downstream of the weirs, but only Kf showed a significant variation (Table 3).
3.5. P Release Rates from Sediment Core Incubation ExperimentsThe incubation of sediments with stream waters at pH 7.0 (±0.5) and 25°C under oxic/anoxic conditions showed that SRP release rates under anoxic conditions increased significantly (DO < 0.5 mg/L) and were significantly higher in upstream sediments compared to downstream sediments of the weirs (Table 4 and Fig. 5). Such discrepancy in the magnitude of SRP release fluxes between the sediments over the weirs did not occur under oxic condition (DO > 7 mg/L) (Fig. 5). The SRP release rates estimated under oxic conditions of the water columns showed significant relationships with Db (r = −0.842; p < 0.05), TP (r = 0.822; p < 0.05), and H2SO4-extractable P (r = 0.973; p < 0.01) (Table 5). On the other hand, in addition to the relations with bulk density (r = −0.969; p < 0.01) and TP (r = 0.918; p < 0.01), SRP release rates under anoxic condition also showed significant relationships with NaOH-extractable P (r = 0.817; p < 0.05), Fe (r = 0.845; p < 0.05), Al (r =0.887; p < 0.05), and Mg concentrations (r = 0.886; p < 0.05) (Table 5).
The SRP release rates under different water pH, temperature, and DO conditions in 18 sediment cores collected from one randomly-chosen location are shown in Fig. 6. SRP release rates were greater under anoxic condition at higher temperature and un-neutral pH. Specifically, the impacts of DO and temperature were illustrated clearly with the incubation experiments at pH 7.0 (Fig. 6(b)). Under oxic condition and neutral pH, P was sufficiently trapped within the sediments at 15°C, while an immediate release of SRP occurred when the temperature increased to 25°C. The SRP release rates at 25°C under anoxic condition were also discernibly higher than those under oxic condition at neutral pH. On the other hand, the SRP release of sediments showed complicated patterns in response to different pH levels. SRP release rates were greater at pH 3.0 and 11.0 compared to pH 7.0 (Fig. 6). The variations of SRP release rates at un-neutral pH strongly depended on the interacting effects of temperature and DO (Fig. 6(a) and 6(c)).
4. DiscussionSRP concentrations in stream water were consistently high (> 0.3 mg/L) and did not vary across the weirs in Tanchun Stream. Urban streams are usually described with elevated concentrations of nutrients and contaminants [42]. Phosphorus concentrations approaching 0.3 mg/L greatly enhance the growth of macrophytes and algal communities [43]. Thus, potentially increasing internal loading of P from streambed sediments upstream of weirs could lead to streams becoming eutrophic.
The lower Db of sediments upstream of weirs, compared to downstream sediments, indicated that the upstream sediments might be more susceptible to resuspension than downstream sediments; thus necessarily enabling P release into the water column [44]. Organic-rich sediments usually have lower Db and contain higher phosphorus concentration [45]. The Fe, Al, Mg, and Ca ions associated the organic matter in sediments tend to retain P by adsorption on surfaces or by forming insoluble complexes and precipitates [45]. We found that the amounts of organic matter and TP, as well as total metals (Fe, Al, Mg, and Ca), were significantly greater in sediments upstream of weirs. Correlation analysis also demonstrated that TP concentrations were associated with organic matter and metallic ions in sediments. Furthermore, the significant positive correlation between TSS and SOM, as well as TP, suggests that weirs acted as important physical barriers to the downstream transport of P in streams.
The significant difference of total P concentrations between sediments upstream and downstream of weirs was mainly due to the differences in P adsorbed and precipitated with Fe and Al in sediments. Labile-P contributed only a small portion of the variation of TP concentrations in sediments across the weirs. Frequent transports of sediments occur during moderate flows in streams [46]. In addition, Fe and Al-bound P fractions in a wide range of sediments are most concentrated near the sediment surface [47–49]. Therefore, any change of stream flows, such as those across weirs, could strongly affect the transport of surface sediments, thus of Fe and Al-bound P fractions. TSS concentrations significantly correlated with NH4F-extractable P and NaOH-extractable P fractions, providing further evidence that the retention of P in streambed sediments were strongly depending on the sediment transport and hydrological regime in streams. These results also implied that the alteration of hydrology and consequent sediment transport could lead to the enrichment of potentially-labile P fractions in streambed sediments of urban streams. If phosphorus is retained in sediments in the potentially available forms, such as phosphorus associated with Fe and Al, it may constitute a significant source to the water column under certain changes of redox potential and pH [22, 50]. Thus, the majority of P pools in sediments trapped above the weirs, which was composed of labile, Fe and Al-bound P forms, would be fairly unstable and potentially affect the stream water quality. Potential release of P from sediments is most likely to occur in the summer, when oxygen demand and primary productivity are highest [22], or at stream sites with elevated organic matter during low (or pulse) flow conditions [51].
Extracellular enzyme activities were often used to measure the limitation of nutrients in ecosystems [52, 53]. In this study, we used these parameters as indicators of the microbial processing of P in surface sediments. The significantly higher activities of β-glucosidase and phosphatase in sediments upstream of the weirs, compared to downstream sediments, indicated the high oxidation potential of organic matter and reduction of various oxidants. The SRP concentrations in the interstitial water and the water column are assumed to be controlled partly by the mineralization of organic matter in sediments. During the mineralization of organic matter in sediments, bacteria consumed O2, NO3−, and SO42− and provided the necessary conditions for the abiotic or biotic reduction of ferric ion (Fe3+), the subsequent release of P, and precipitation of iron sulfide (FeS) [54].
The relative contribution of abiotic or biotic processes to the P retention and remobilization in sediments upstream of weirs might also be changed compared to natural streambed sediments (in sensu Lottig and Stanley [55]). The role of biotic processes controlling the SRP concentration in the interstitial water of sediments is often assumed to be limited [54]. However, Lottig and Stanley [55] demonstrate that the type and strength of sediment-associated processes determining the SRP concentrations in sediments are potentially altered due to changes in sediment composition (i.e., fine versus coarse particles). Accordingly, we propose that the P remobilization of sediments with higher silt and clay contents above the weirs will be dominated by abiotic processes, whereas biotic processes will mainly account for the P remobilization in naturally sandy sediments. Over time, if the abiotic P sink of sediments above the weirs is filled up due to the increase of P loads and the absence of scouring floods, the sediments above the weirs will shift to release P. The biotic P sink in natural sediments, however, can be refreshed with frequently new sediment input. Therefore, as the weirs can limit the sediment turnover in streams, they can also diminish long-term P buffering capacity of streams. This hypothesis needs to be evaluated in further experiments.
The EPCo of sediments lower than the ambient SRP concentrations demonstrated that the sediments were still able to retain phosphorus. However, the buffer intensities of sediments with respect to any external loading of dissolved phosphorus significantly changed across the weirs. The lower Kd (or lower Kf) and the higher EPCo of sediments upstream of the weirs indicated that sediments above weirs served as a less sustained sink of phosphorus to the water column. Sorption behavior of sediments in respect to Kd (or Kf) and EPCo was argued to relate to the total exchangeable P pool of sediments [44]. It is assumed that organic P contributed less to the exchangeable P pool of our sediments, which is possibly reasonable in this study because the residual P has accounted for only 10% of the total P of sediments. The total amount of exchangeable P in our sediments can be calculated as a sum of NH4Cl-, NH4F-, NaOH- and CDB-extractable P fractions. As a result, the mean exchangeable P concentration is 357.966 ± 44.383 mg/kg of upstream sediments, which is higher than that of downstream sediments (116.029 ± 13.295 mg/kg). Furthermore, we found from the batch experiments that NAP concentrations were significantly higher in sediments upstream of weirs compared to downstream sediments. Thus, our results are consistent with Zhang and Huang’s finding [44] suggesting that the lower buffering intensity of sediments upstream of weirs is due to the saturation of exchangeable P on sediment surfaces. We argue that the presence of weirs could increase P retention in streams and further external loading of P might place the local streams at higher risks of eutrophication. More importantly, if the majority of such very large stores of P upstream of weirs is not exported from the catchments during the highest flow, as has been observed in other populated river catchments [11, 56, 57], the downstream risk of eutrophication will certainly occur [58].
The lack of difference in SRP release rates between sediments across the weirs under in situ mimicking oxic condition suggests that weirs may not obviously affect the ambient P concentration of streams under natural conditions with the high mixing in the water column. A significant increase of SRP release from sediments above the weirs, compared to downstream sediments, could occur when the overlying water was switched from oxic to anoxic condition (DO < 0.5 mg/L). The kinetic experiments of P release in intact sediment cores demonstrate that the P release rate is further a function of temperature and pH. P retaining effect of oxic sediments was well-observed in previous studies and was attributed to the coupling between iron (Fe) and P cycles in a micro-layer at the sediment-water interface [20]. However, temperature, in interaction with DO, affects the P release in sediments in at least two ways. Firstly, higher temperature increases the mineralization rate of easily degradable organic material. Secondly, higher oxygen demands for mineralization in turn lower the amount of available oxygen in sediments [20]. On the other hand, pH changes cause different effects on the P-binding capacity of sediments. The P-binding capacity of Fe (and Al) oxides in sediments decreases under alkaline pH condition, primarily due to the replacement between OH− and PO43− in ligand-exchange reactions, which can lead to P releases from sediments. Alkaline pH is also a precondition for the co-precipitation of P in the water, which can promote reversed P fluxes into sediments [20]. A decrease of pH can also occur as a result of CO2 production following the mineralization in sediments which can lead to the solubilization of settled calcite and apatite P [20]. Overall, the response of sediments (in term of P remobilization) to different manipulations of DO, temperature and pH in the water column has the important implications for the water management in urban streams. Changing physicochemical properties of stream water as a result of the impoundment with weirs could promote a further positive feedback on P release in streams [19]. In addition, unmanaged effluent releases from combined waste-water treatment plants, probably during high rainfall periods, along the Tanchun Stream and other urban streams that have weirs should be carefully investigated and restricted to avoid acute short-term loadings of P and variations of water physicochemical conditions, which are possibly harmful to the receiving streams [59].
Our study further emphasized that Fe-Al-P interactions are important to P dynamics in non-calcareous sediments. The anoxic SRP release rates were significantly correlated to TP and NaOH-extractable P fraction, as well as to total Fe and Al concentrations. The role of sediment TP and metals in determining P release fluxes was widely reported [60–63]. However, it is important to note that, rather than Fe-P appearing predominantly in calcareous sediments, Fe-Al-P was predominantly in non-calcareous sediments [64]. Compared to Al-bound P, Fe-bound P was considered as more potentially labile [65]. High concentrations of aluminum hydroxides in sediments could even prevent the P release [66]. This implies that if the sediments trapped above weirs are calcareous sediments containing the same amounts of P, they may contribute to a higher risk of eutrophication in streams.
5. ConclusionsWeirs could increase P retention in urban streams. Phosphorus was mostly retained in sediments above weirs in the exchangeable forms, which potentially constituted a significant P source to waters under changing environmental conditions. Remobilization of sediment P above weirs thus can promote eutrophication in urban streams. Since interactions between sediments and stream water are highly heterogeneous, quantifying the significance of impoundment effects on P retention and remobilization needs to be intensified to gain valuable insights into the P transport and transformation as moving through watersheds.
AcknowledgmentsThis study was supported by the Center for Aquatic Ecosystem Restoration (CAER) of the Eco-STAR project from the Ministry of Environment, Republic of Korea (No. EW33-08-11) and by the National Research Foundation of Korea (No. 20110029802).
References1. Duan S, Kaushal SS, Groffman PM, Band LE, Belt KT. (2012)Phosphorus export across an urban to rural gradient in the Chesapeake Bay watershed. J. Geophys. Res. Biogeosci. (2005–2012). 2012;117:G01025.
2. Burford MA, Green SA, Cook AJ, Johnson SA, Kerr JG, O’Brien KR. Sources and fate of nutrients in a subtropical reservoir. Aquat. Sci. 2012;74:179–190.
3. Torres IC, Resck RP, Pinto-Coelho RM. Mass balance estimation of nitrogen, carbon, phosphorus and total suspended solids in the urban eutrophic, Pampulha reservoir, Brazil. Acta Limnologica Brasiliensia. 2007;19:79–91.
4. Palmer-Felgate EJ, Jarvie HP, Williams RJ, Mortimer RJ, Loewenthal M, Neal C. Phosphorus dynamics and productivity in a sewage-impacted lowland chalk stream. J. Hydrol. 2008;351:87–97.
5. Carpenter SR. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10002–10005.
6. Schindler DW. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006;51:356–363.
7. Ackers P, White W, Perkins JA, Harrison AJ. Weirs and flumes for flow measurement. Chichester: John Wiley & Sons; 1978.
8. Praskievicz S, Chang H. A review of hydrological modelling of basin-scale climate change and urban development impacts. Prog. Phys. Geogr. 2009;33:650–671.
9. Hassan MA, Church M, Lisle TE, Brardinoni F, Benda L, Grant GE. Sediment transport and channel morphology of small, forested streams. J. Am. Water Resour. Assoc. 2005;41:853–876.
10. Kröger R, Moore MT, Farris JL, Gopalan M. Evidence for the use of low-grade weirs in drainage ditches to improve nutrient reductions from agriculture. Water Air Soil Pollut. 2011;221:223–234.
11. Demars BO, Harper DM, Pitt JA, Slaughter R. Impact of phosphorus control measures on in-river phosphorus retention associated with point source pollution. Hydrol. Earth Syst. Sci. 2005;2:43–55.
12. US Environmental Protection Agency. Federal Water pollution Control Act: as amended through P.L. 107–303, November 27, 2002 [Internet]. Washington: US Environmental Protection Agency; 2002. [cited 2014 May 26]. Available from: http://www.epw.senate.gov/water.pdf
13. Usborne EL, Kröger R, Pierce SC, Brandt J, Goetz D. Preliminary evidence of sediment and phosphorus dynamics behind newly installed low-grade weirs in agricultural drainage ditches. Water Air Soil Pollut. 2013;224:1–11.
14. Moss B, Balls H, Booker I, Manson K, Timms M. Problems in the construction of a nutrient budget for the River Bure and its Broads (Norfolk) prior to its restoration from eutrophication. Lund JW, Round FE, editorsAlgae and the aquatic environment. Bristol: Biopress; 1988. p. 327–353.
15. Sharpley A, Jarvie HP, Buda A, May L, Spears B, Kleinman P. Phosphorus legacy: overcoming the effects of past management practices to mitigate future water quality impairment. J. Environ. Qual. 2013;42:1308–1326.
16. James WF, Larson CE. (2008)Phosphorus dynamics and loading in the turbid Minnesota River (USA): controls and recycling potential. Biogeochem. 2008;90:75–92.
17. McDowell RW, Sharpley AN, Chalmers AT. Land use and flow regime effects on phosphorus chemical dynamics in the fluvial sediment of the Winooski River, Vermont. Ecol. Eng. 2002;18:477–487.
18. Kleinman P, Sharpley A, Buda A, Mcdowell R, Allen A. Soil controls of phosphorus in runoff: management barriers and opportunities. Can. J. Soil Sci. 2011;91:329–338.
19. Rickard C, Day R, Purseglove J. River weirs: good practice guide. Bristol: Environment Agency; 2003.
20. Hupfer M, Lewandowski J. Oxygen controls the phosphorus-release from lake sediments: a long-lasting paradigm in limnology. Int. Rev. Hydrobiol. 2008;93:415–432.
21. Utley BC, Vellidis G, Lowrance R, Smith MC. Factors affecting sediment oxygen demand dynamics in blackwater streams of Georgia’s coastal plain. J. Am. Water Resour. Assoc. 2008;44:742–753.
22. Moore A, Reddy KR. Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida. J. Environ. Qual. 1994;23:955–964.
23. Reddy KR, Diaz OA, Scinto LJ, Agami M. Phosphorus dynamics in selected wetlands and streams of the Lake Okeechobee Basin. Ecol. Eng. 1995;5:183–207.
24. Mortimer CH. Chemical exchanges between sediments and water in the Great Lakes-speculations on probable regulatory mechanisms. Limnol. Oceanogr. 1971;16:387–404.
25. Mortimer CH. The exchange of dissolved substances between mud and water in lakes. J. Ecol. 1941;29:280–329.
26. Mortimer CH. The exchange of dissolved substances between mud and water in lakes. J. Ecol. 1942;30:147–201.
27. Gächter R, Müller B. Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. Limnol. Oceanogr. 2003;48:929–933.
28. Gächter R, Wehrli B. Ten years of artificial mixing and oxygenation: no effect on the internal phosphorus loading of two eutrophic lakes. Environ. Sci. Technol. 1998;32:3659–3665.
29. Blomqvist S, Gunnars A, Elmgren R. Why the limiting nutrient differs between temperate coastal seas and freshwater lakes: a matter of salt. Limnol. Oceanogr. 2004;49:2236–2241.
30. Caraco NF, Cole JJ, Likens GE. Evidence for sulphate-controlled phosphorus release from sediments of aquatic systems. Nature. 1989;341:316–318.
31. Korean Ministry of Environment. Statistics of sewerage 2006. Gwacheon: Ministry of Environment; 2007.
32. Greenberg AE, Clesceri LS, Eaton AD. Standard methods for the examination of water and wastewater. 18th edWashington: American Public Health Association; 1992.
33. Page AL. Methods of soil analysis: Part 2. Chemical and microbiological properties. Madison: American Society of Agronomy, Soil Science Society of America; 1982.
34. Kang H, Freeman C, Lee D, Mitsch WJ. Enzyme activities in constructed wetlands: implication for water quality amelioration. Hydrobiologia. 1998;368:231–235.
35. Aran D, Maul A, Masfaraud JF. A spectrophotometric measurement of soil cation exchange capacity based on cobaltihexamine chloride absorbance. Comptes Rendus Geosci. 2008;340:865–871.
36. Chen M, Ma LQ. Comparison of four USEPA digestion methods for trace metal analysis using certified and Florida soils. J. Environ. Qual. 1998;27:1294–1300.
37. Zhang H, Kovar JL. Fractionation of soil phosphorus. Pierzynski GM, editorMethods of phosphorus analysis for soils, sediments, residuals, and waters. North Caroline: North Carolina State University; 2009. p. 50–60.
38. Vo NX, Ji YH, Kang HJ. Distribution of phosphorus fractions in the sediment of South Han River during a rainy season. In : Proceedings of the 2012 World Congress on Advances in Civil, Environmental, and Materials Research; 2012 Aug 26–30; Seoul, Korea.
39. Auer MT, Johnson NA, Penn MR, Effler SW. Measurement and verification of rates of sediment phosphorus release for a hypereutrophic urban lake. Hydrobiologia. 1993;253:301–309.
40. Taylor AW, Kunishi HM. Phosphate equilibria on stream sediment and soil in a watershed draining an agricultural region. J. Agric. Food Chem. 1971;19:827–831.
41. Jarvie HP, Jürgens MD, Williams RJ, et al. Role of river bed sediments as sources and sinks of phosphorus across two major eutrophic UK river basins: the Hampshire Avon and Herefordshire Wye. J. Hydrol. 2005;304:51–74.
42. Meyer JL, Paul MJ, Taulbee WK. Stream ecosystem function in urbanizing landscapes. J. North Am. Benthol. Soc. 2005;24:602–612.
43. Mainstone CP, Parr W. Phosphorus in rivers: ecology and management. Sci. Total Environ. 2002;282:25–47.
44. Zhang JZ, Huang XL. Relative importance of solid-phase phosphorus and iron on the sorption behavior of sediments. Environ. Sci. Technol. 2007;41:2789–2795.
45. Belmont MA, White JR, Reddy KR. Phosphorus sorption and potential phosphorus storage in sediments of Lake Istokpoga and the upper chain of lakes, Florida, USA. J. Environ. Qual. 2009;38:987–996.
46. Erskine WD, Saynor MJ. Hydrology and bedload transport relationships for sand-bed streams in the Ngarradj Creek catchment, northern Australia. J. Hydrol. 2013;483:68–79.
47. Han L, Huang S, Stanley CD, Osborne TZ. Phosphorus fractionation in core sediments from Haihe River mainstream, China. Soil Sediment Contam. 2011;20:30–53.
48. Jensen HS, Mortensen PB, Andersen FO, Rasmussen E, Jensen A. Phosphorus cycling in a coastal marine sediment, Aarhus Bay, Denmark. Limnol. Oceanogr. 1995;40:908–917.
49. Moore PA, Reddy KR, Fisher MM. Phosphorus flux between sediment and overlying water in Lake Okeechobee, Florida: spatial and temporal variations. J. Environ. Qual. 1998;27:1428–1439.
50. Olila OG, Reddy KR. Influence of pH on phosphorus retention in oxidized lake sediments. Soil Sci. Soc. Am. J. 1995;59:946–959.
51. Diaz OA, Daroub SH, Stuck JD, Clark MW, Lang TA, Reddy KR. Sediment inventory and phosphorus fractions for water conservation area canals in the Everglades. Soil Sci. Soc. Am. J. 2006;70:863–871.
52. Kang H, Kim SY, Fenner N, Freeman C. Shifts of soil enzyme activities in wetlands exposed to elevated CO2. Sci. Total Environ. 2005;337:207–212.
53. Vo NX, Kang H. Regulation of soil enzyme activities in constructed wetlands under a short-term drying period. Chem. Ecol. 2013;29:146–165.
54. Gächter R, Meyer JS. The role of microorganisms in mobilization and fixation of phosphorus in sediments. Hydrobiologia. 1993;253:103–121.
55. Lottig NR, Stanley EH. Benthic sediment influence on dissolved phosphorus concentrations in a headwater stream. Biogeochemistry. 2007;84:297–309.
56. Dorioz JM, Cassell EA, Orand A, Eisenman KG. Phosphorus storage, transport and export dynamics in the Foron River watershed. Hydrol. Process. 1998;12:285–309.
57. Svendsen LM, Kronvang B. Retention of nitrogen and phosphorus in a Danish lowland river system: implications for the export from the watershed. Hydrobiologia. 1993;251:123–135.
58. Jarvie HP, Neal C, Withers PJ, Baker DB, Richards RP, Sharpley AN. (2011)Quantifying phosphorus retention and release in rivers and watersheds using extended end-member mixing analysis (E-EMMA). J. Environ. Qual. 2011;40:492–504.
59. Burian SJ, Nix SJ, Pitt RE, Durrans SR. Urban wastewater management in the United States: past, present, and future. J. Urban Technol. 2000;7:33–62.
60. Boström B, Andersen JM, Fleischer S, Jansson M. Exchange of phosphorus across the sediment-water interface. Hydrobiologia. 1988;170:229–244.
61. Das J, Daroub SH, Bhadha JH, Lang TA, Josan M. Phosphorus release and equilibrium dynamics of canal sediments within the Everglades Agricultural Area, Florida. Water Air Soil Pollut. 2012;223:2865–2879.
62. Nürnberg GK. Prediction of phosphorus release rates from total and reductant-soluble phosphorus in anoxic lake sediments. Can. J. Fish. Aquat. Sci. 1988;45:453–462.
63. Williams JD, Syers JK, Armstrong DE, Harris RF. Characterization of inorganic phosphate in noncalcareous lake sediments. Soil Sci Soc Am J. 1971;35:556–561.
64. Zanini L, Robertson WD, Ptacek CJ, Schiff SL, Mayer T. Phosphorus characterization in sediments impacted by septic effluent at four sites in central Canada. J. Contam. Hydrol. 1998;33:405–429.
Fig. 1Location of study sites in Tancheon stream, a tributary of the Han River. Two free overfall weirs were surveyed and sampled in May 2012. 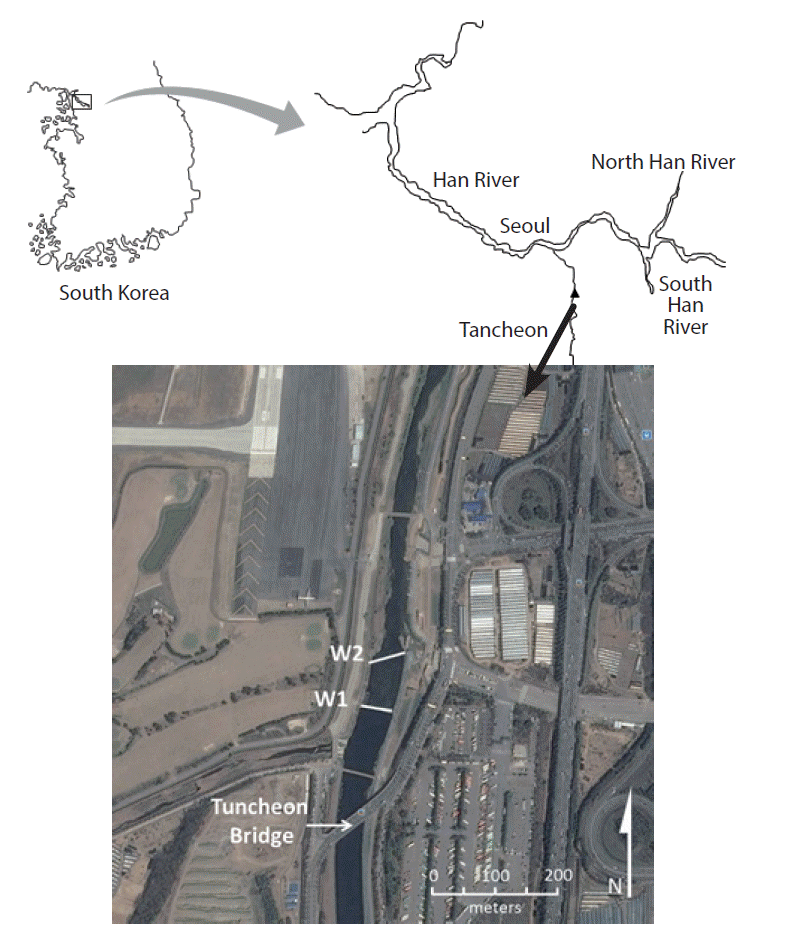
Fig. 2Freundlich isotherm fitting of equilibrium adsorption-desorption data (see the text for notations). EPCo: Equilibrium phosphorus concentration, NAP: native adsorbed phosphorus. 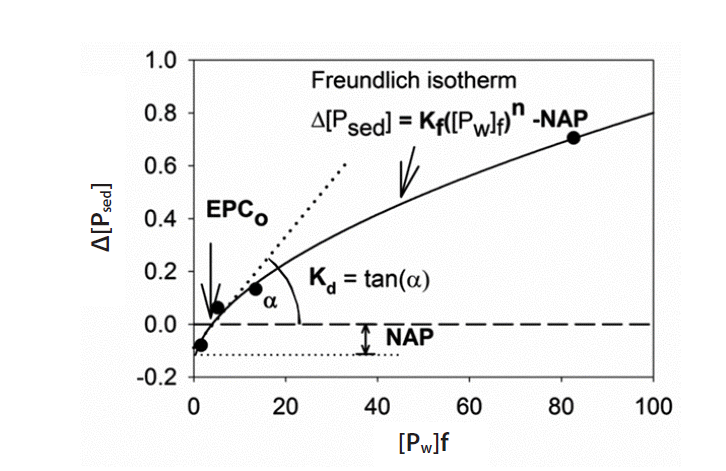
Fig. 3Spatial distribution of (a) total concentration of metals and (b) extracellular enzyme activities in sediments across weirs (mean ± SE; n = 3). Asterisks denote significant differences between upstream and downstream sediments at *p < 0.05, **p < 0.01, ***p < 0.001. 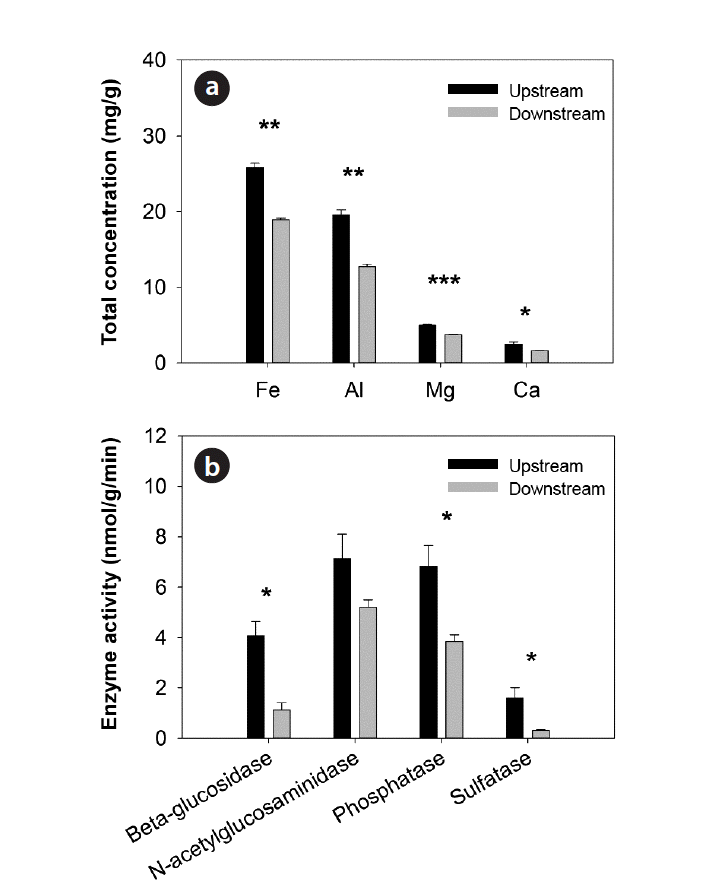
Fig. 4Distribution of P fractions in sediments across weirs (mean ± SE, n = 3). Asterisks denote significant differences between upstream and downstream sediments at *p < 0.05, **p < 0.01, ***p < 0.001. 
Fig. 5(a) Cumulative soluble reactive phosphorus (SRP) releases and (b) SRP release rates in sediments across weirs at 25°C, water pH 7.0 (± 0.5) under oxic/anoxic conditions. The dashed line indicates the switch from oxic (DO > 7 mg/L) to anoxic condition (DO < 0.5 mg/L) in water columns of sediment cores. 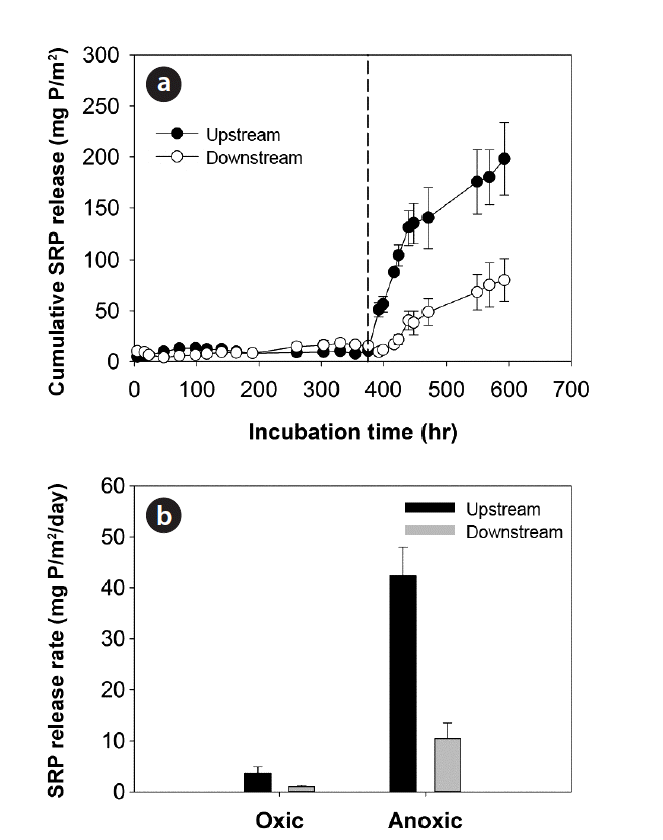
Fig. 6Soluble reactive phosphorus (SRP) release rates (mean ± SE, n = 2) at different oxic/anoxic conditions, temperatures (15°C and 25°C), and pH: (a) pH 3.0, (b), pH 7.0, and (c) pH 11.0. 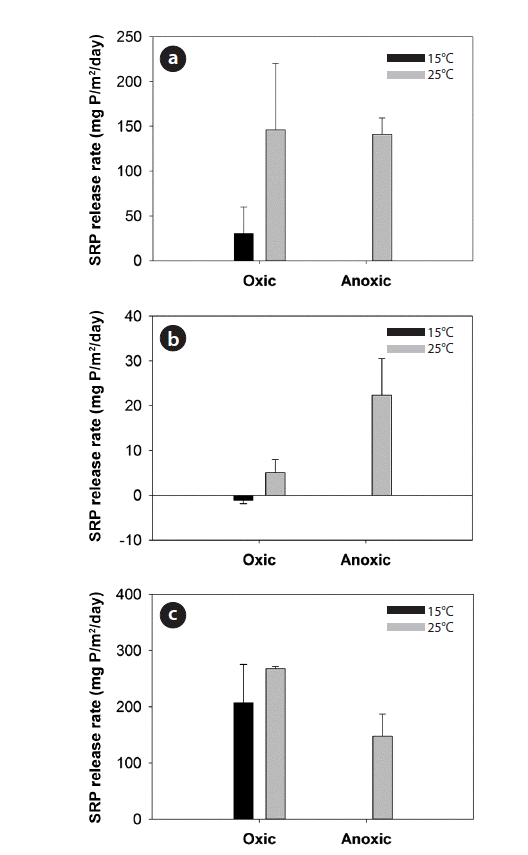
Table 1Physicochemical characteristics of stream water and sediments (n = 3)
Table 2Percent contribution of P fractions to total P in sediments across weirs (n = 3)
Table 3Empirical determination factors of the Freundlich isotherm for sediments across weirs (n = 3)
Table 4Repeated-measures ANOVA for SRP release rates in sediments across weirs
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||