1. Introduction
Groundwater resources are widely used as drinking water supplies around the globe. Therefore, the protection of groundwater from viral contamination has received considerable attention [1]. Manure application to agricultural lands, septic tank effluents released into soils, and wastewater discharged to the vadose zone can lead to viral contamination of groundwater [2–4]. Clay minerals are responsible for sorption and ion exchange processes that occur in soils. In order to understand the role that clays play in the adsorption of viruses in soils, several researchers have tested clays, such as kaolinite, montmorillonite, and bentonite, for virus removal [5–9]. Chattopadhyay and Puls [10] studied the thermodynamics for adsorption of bacteriophages T2, MS2, and PhiX174 to clay minerals, such as kaolinite, hectorite, saponite, and clay fraction, from a landfill site. They demonstrated that bacteriophage sorption to clay particles depends on the surface hydrophobicities of the bacteriophages and clays. Syngouna and Chrysikopoulos [11] investigated the interaction of bacteriophages MS2 and PhiX174 with kaolinite and bentonite. They determined that bacteriophage adsorption to clay increased with an increase of temperature in most cases.
Clays have been used as soil amendment in order to reduce the transfer of contaminants from soils to crops and other media (e.g., groundwater) [12]. Jones et al. [13] used clays, such as bentonite and clinoptilolite, to immobilize radiocaesium (137Cs) in soils and to reduce soil-to-plant transfer of 137Cs. They showed that a significant reduction in soil-to-plant transfer of 137Cs could be achieved in contaminated soils treated with clinoptilolite. García-Sanchez et al. [14] applied clays (bentonite and limonite) and metal oxides (iron hydroxide and aluminum hydroxide) to immobilize arsenate in soils. They reported that the efficiencies of clays in the immobilization of As(V) in the contaminated mining soils were far lower than those of metal oxides. Ling et al. [15] used bentonite to reduce the release of copper (Cu2+) from soils. They reported that the amendment of bentonite effectively decreased the release of Cu2+ from the contaminated soils and the magnitude of Cu2+ immobilization increased with an increase in the amount of bentonite applied to the soils.
In South Korea, a serious outbreak of foot and mouth disease (FMD) occurred in 2010–2011, leading to the massive burial of farm animals (pigs and cattle) in forty-six hundred locations around the nation. People were concerned about the microbial contamination of groundwater due to leachates from the animal carcass burial sites; therefore, they became interested in countermeasures to reduce the transfer of microorganisms from the burial sites to groundwater.
The aim of this study was to investigate the bacteriophage removal in various clay minerals and clay-amended soils. First, the removals of MS2 in three clay minerals (kaolinite, montmorillonite, and bentonite) were compared using batch experiments. Based on the first experiments, the removals of MS2 in soils amended with various contents of kaolinite were studied in the second batch experiments at three different soil-to-solution ratios. In the third experiments, batch experiments were further extended to compare the removals of PhiX174 and Qβ with the removals of MS2 in soils amended with various contents of kaolinite.
2. Materials and Methods
2.1. Preparation of Soil and Clay Minerals
Silt loam soil collected from a carcass burial site in Umsung, Korea, was used in this experiment. The soil was air-dried, passed through US Standard Sieves No. 100, and sterilized in an autoclave. The characteristics of the soil are presented in Table 1. The soil had a pH of 5.24, a cation exchange capacity (CEC) of 8.13 cmol kg−1, and 3.99% organic matter. It also contained 33.77% sand, 58.67% silt, and 7.56% clay.
Clay minerals, such as kaolinite (Fluka No: 03584), montmorillonite (Fluka No: 69866), and bentonite (Sigma Aldrich No: 285234), were used in this study. Kaolinite (Al2O3 · 2SiO2· 2H2O) is a 1:1 (Si:Al) non-expanding clay, whereas montmorillonite (Al2O3 ·4SiO2·nH2O) and bentonite (Al2O3·4SiO2·2H2O) are 2:1 (Si:Al) expanding clays. In order to obtain uniform particle size of the clays, the clays were prepared using the procedures described in the literature [16]. The clays were suspended in deionized water, ultrasonically dispersed for 30 min, and then allowed to settle for 24 h. After settling, the supernatant was siphoned out in order to obtain clay particles with diameters < 2 μm according to the calculations based on Stoke’s law [16]. The clay particles in the supernatant were passed through a 0.1 μm membrane filter in order to obtain the clay particles used in this study. Prior to use, the clay particles were oven-dried for 18 h at 65°C.
2.2. Preparation of Bacteriophages
The bacteriophages MS2 (ATCC 15597-B1), PhiX174 (ATCC 13706-B1), and Qβ (ATCC 23631-B1), obtained from the American Type Culture Collection, were used as an indicator of the human enteric virus [17]. Both MS2 and Qβ are F-specific and unenveloped single-stranded RNA phages, whereas PhiX174 is a somatic and single-stranded DNA phage [18]. MS2, PhiX174, and Qβ were grown on Escherichia coli (ATCC 15597), Escherichia coli C (ATCC 13706), and Escherichia coli (ATCC 23631), respectively, using the double agar overlay method [18]. The bacteriophages were enumerated using the plaque assay method with the aforementioned hosts. The host culture (0.2 mL) and 0.1 mL of a diluted virus sample with 5 mL of soft agar were added to tubes, and then the mixture was poured onto trypticase soy agar (TSA) plates to solidify. After solidifying, the plates were incubated at 37°C for 18 h.
2.3. Bacteriophage Removal Experiments
Batch experiments were conducted in triplicate to examine the removal of bacteriophages in the clays and kaolinite-amended soils. The bacteriophage stock solution was diluted from a concentrated titer with an artificial ground water (AGW; 0.075 mM CaCl2, 0.082 mM MgCl2, 0.051 mM KCl, 1.5 mM NaHCO3, pH 7.6) to the desired concentration (105–106 pfu mL−1). The first set of batch experiments was performed in order to examine the removal of MS2 as a function of the adsorbent (kaolinite, montmorillonite, and bentonite) dosage. The experimental method consisted of adding 50 mL virus stock solution to 50 mL centrifuge tubes containing different dosages of the adsorbent (0.3–50 g L−1). After all of the tubes were properly prepared and sealed, they were shaken at 100 rpm for 240 min at 4°C in order to avoid thermal inactivation of the virus. Control tubes with no clay particles were used to monitor the virus inactivation. The suspensions were then centrifuged at 9,000 × g and 4°C for 15 min (Combi-514R; Hanil Science Industrial, Incheon, Korea). The viable bacteriophage concentration was determined using the plaque assay method. The control tubes were filled with only bacteriophage solution and treated in the same manner as the experimental tubes. In the second set of experiments, kinetic tests were conducted in 50 mL centrifuge tubes in order to observe the removal of MS2 by kaolinite (kaolinite dose = 50 g L−1). The tubes were shaken at 100 rpm for a set of desired reaction times ranging from 5 to 240 min.
The third set of experiments was conducted in 500 mL flask in order to examine the removal of MS2 by kaolinite-amended soils (kaolinite content in soil = 0–10 wt.%) at three different soil-to-solution (STS) ratios (1:10, 1:2, and 1:1) with a reaction time of 240 min. The STS ratios of 1:10, 1:2, and 1:1 are equivalent to the adsorbent doses of 100, 500, and 1,000 g L−1, respectively. The bacteriophages were enumerated by the same experimental procedures as the first experiments were followed. The fourth set of experiments was performed in 500 mL flask in order to compare the removal rates of other bacteriophages PhiX174 and Qβ in kaolinite-amended soils (kaolinite content in soil = 0–10 wt.%) with the removal rate of MS2. The same experimental conditions as the third experiment were used in the experiments with a STS ratio of 1:10 and a reaction time of 240 min.
The bacteriophage removal was calculated using the following formula:
where R is the percent removal of bacteriophage, and C0 and C are the initial and final bacteriophage concentrations, respectively. The log removal of the bacteriophage was calculated using the following relationship:
The bacteriophage removal per unit mass of adsorbent was calculated using the following formula:
where S is the amount of bacteriophage removed per one gram of adsorbent, and M is the adsorbent concentration used in the experiment.
3. Results and Discussion
3.1. MS2 Removal in Kaolinite, Montmorillonite, and Bentonite
The log removal and removal capacity of the bacteriophage MS2 in kaolinite, montmorillonite, and bentonite as a function of the adsorbent dose are presented in Fig. 1. In kaolinite, the log removal increased from 0.046 to 2.18, whereas the removal capacity decreased from 9.75 × 108 to 6.44 × 107 pfu g−1 as the kaolinite dose increased from 0.3 to 50 g L−1. The log removal in the montmorillonite increased slightly from 0.007 to 0.40, whereas the removal capacity decreased from 1.50 × 108 to 3.87 × 107 pfu g−1 with an increase in the montmorillonite dose from 0.3 to 50 g L−1. In bentonite, the log removal changed from 0.012 to 0.59, whereas the removal capacity decreased from 2.70 × 108 to 4.56 × 107 pfu g−1 with an increase in the bentonite dose from 0.3 to 50 g L−1. At the same dose of adsorbent, the log removal and removal capacity of MS2 was the highest in kaolinite among the three clay minerals.
The characteristics of the clays used in the experiments are presented in Table 2. Kaolinite has a chemical composition of SiO2 (49.0%), Al2O3 (34.7%), K2O (2.2%), and Fe2O3 (0.4%), with a BET specific surface area of 9 m2 g−1 and a CEC of 6.2 cmol kg−1 [19]. Montmorillonite has a chemical composition of SiO2 (55.0%), Al2O3 (18.0%), SO3 (5.0%), and Fe2O3 (4.0%) with a BET specific surface area of 250 m2 g−1 and a CEC of 86 cmol kg−1 [20, 21]. Bentonite is composed of SiO2 (58.3%), Al2O3 (18.9%), Fe2O3 (4.0%), and Na2O (2.3%), and has a BET specific surface area of 32.6 m2 g−1 and a CEC of 110 cmol kg−1 [22]. The specific surface area of the clays was in the order of kaolinite < bentonite < montmorillonite. In addition, the CEC of the clays was in the order of kaolinite < montmorillonite < bentonite.
Our results demonstrated that kaolinite was far more effective in the removal of MS2 than montmorillonite and bentonite, even though the specific surface area and CEC of kaolinite was far lower than those of the montmorillonite and bentonite. Similar findings were reported in the literature by Chrysikopoulos and Syngouna [23], who performed batch experiments in order to examine the adhesion of MS2 and PhiX174 to kaolinite (KGa-1b) and bentonite (STx-1b). They reported that the adhesion of MS2 and PhiX174 was greater to kaolinite than bentonite, even though the specific surface area (10.1 m2 g−1) and CEC (2.0 cmol kg−1) of kaolinite were far smaller than those (82.9 m2 g−1, 84.4 cmol kg−1) of bentonite.
Moore et al. [24] also reported from batch experiments that the adsorption percentages (99.5% and 98.7%) of the poliovirus to two kaolinites were greater than those (91.5% and 94.1%) to two montmorillonites, even though the specific surface areas of kaolinites (16 and 12 m2 g−1) were lower than those of montmorillonites (41 and 32 m2 g−1). Sobsey et al. [25] showed that the adsorption of the hepatitis A virus (HAV) was greater onto kaolinite (99%) than bentonite (28%) in groundwater at a pH level of 7. In addition, the adsorptions of HAV, poliovirus, and echovirus were greater onto kaolinite (all 99%) than onto bentonite (8–36%) in secondary wastewater effluent at a pH level of 7.
Montmorillonite and bentonite have larger specific surface areas than kaolinite because they can swell when exposed to water (Table 2). It was reported that montmorillonite and bentonite are more efficient to adsorb heavy metal ions than kaolinite [26]. In the case of virus, however, high surface area is not positively related to adsorption of virus to clay minerals because nano-sized virus cannot intercalate between layers in expanding clay minerals (10–20 Å) [27, 28]. Instead, the positively-charged edge sites (e.g.≡ Al-OH) on the clays can play a major role for virus adsorption. Kaolinite has higher compositions of aluminum plus iron than montmorillonite and bentonite (Table 2). It was also reported that the total edge area of kaolinite (20–30%) is higher than montmorillonite (< 1%) [29].
3.2. Kinetic and Equilibrium Model Analysis for MS2 Removal in Kaolinite
The kinetic model analysis was performed for the experimental data for MS2 removal in kaolinite (Fig. 2). In the model analysis, the following linear forms of the pseudo first-order and pseudo second-order kinetic models were used:
where qe is the amount of MS2 removed at equilibrium, qt is the amount of MS2 removed at time t, k1 is the pseudo first-order rate constant, and k2 is the pseudo second-order rate constant. The kinetic model parameters are provided in Table 3. The correlation coefficient showed that the pseudo second-order model described the kinetic data well. The amount of MS2 removed at equilibrium (qe) was determined to be 6.58×106 pfu g−1 from the pseudo second-order model.
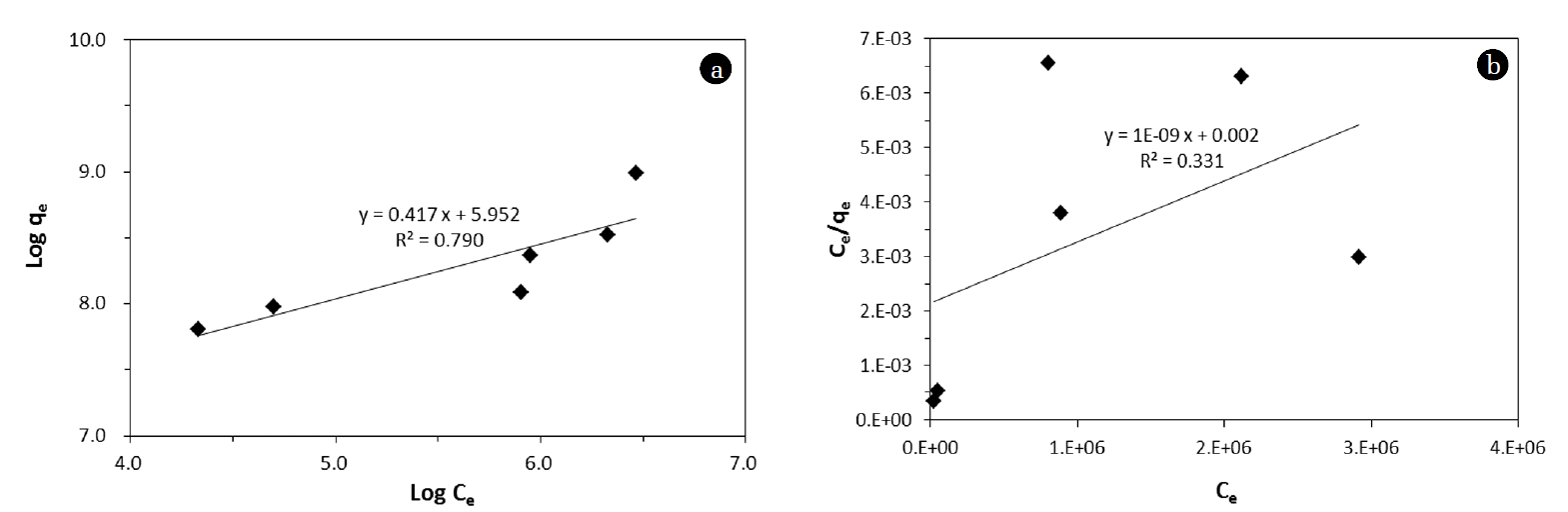
The equilibrium isotherm model analysis was performed for the experimental data from removal in kaolinite (Fig. 3). In the model analysis, the following linear forms of the Freundlich and Langmuir isotherm models were used:
where Ce is the concentration of MS2 in the aqueous solution at equilibrium, KF is the distribution coefficient, 1/n is the Freundlich constant, Qm is the maximum mass of MS2 removed per unit mass of kaolinite (removal capacity), and KL is the Langmuir constant related to the binding energy. The equilibrium model parameters are provided in Table 4. The correlation coefficients showed that the Freundlich model was more suitable than the Langmuir model for MS2 data. The maximum amount of MS2 removed per unit mass of kaolinite (Qm) was determined to be 1.96 × 106 pfu g−1.
3.3. Removal of MS2, PhiX174, and Qβ in Kaolinite-amended Soils
The log removals of MS2 in kaolinite-amended soil under various kaolinite contents and STS ratios are shown in Fig. 4. In the soil without added kaolinite (kaolinite content = 0%), the log removal of MS2 was 2.33 in the STS ratio of 1:10. As the STS ratio increased to 1:2, the log removal increased to 2.84. At the highest STS ratio of 1:1, the log removal further increased to 3.46. These results could be attributed to the fact that more sorption sites are available for MS2 with an increase in the STS ratio. In kaolinite-amended soil (kaolinite content = 3%), the log removal was 2.46 at the STS ratio of 1:10, indicating that MS2 removal increased with an increase in kaolinite content in the soil. At the kaolinite contents of 5% and 7%, the log removals were 2.57 and 2.60, respectively, at the STS ratio of 1:10. At the highest kaolinite content of 10%, the log removal further increased to 2.80. As the STS ratio increased in kaolinite-amended soil, the log removals of MS2 also increased. At the STS ratio of 1:2, the log removal increased from 2.84 to 3.47 with an increase in the kaolinite content from 0% to 10 %. In addition, the log removal increased from 3.46 to 4.76 at the STS ratio of 1:1 with an increase in the kaolinite content from 0% to 10%.
The log removals of MS2, PhiX174, and Qβ in kaolinite-amended soil (STS = 1:10) under various kaolinite contents (0–10%) are compared in Fig. 5. In the soil without added kaolinite (kaolinite content = 0%), the log removals of PhiX174 and Qβ were 0.16 and 0.17, respectively, which were one order of magnitude lower than that of MS2 (2.33). As the kaolinite content increased to 3%, the log removals of PhiX174 and Qβ increased slightly to 0.26 and 0.18, respectively. At the highest kaolinite content of 10%, the log removals of PhiX174 and Qβ further increased to 0.32 and 0.22, respectively. As the kaolinite content in the soil increased, the log removals of bacteriophages also increased.
Our results demonstrated that the log removals of PhiX174 and Qβ in kaolinite-amended soils were similar to each other, but far lower than those of MS2 at all of the kaolinite contents (0–10%). These results could be attributed to the different characteristics of PhiX174 and Qβ compared to MS2. The characteristics of bacteriophages MS2, PhiX174, and Qβ are presented in Table 5. MS2 has a diameter of 24–26 nm with an isoelectric point of 3.9 [30–33]. PhiX174 has a diameter of 25–27 nm with an isoelectric point of 6.6 [34–37]. Qβ has a diameter of 26 nm with an isoelectric point of 5.3 [33, 38, 39]. Even though the three bacteriophages (MS2, PhiX174, and Qβ) had similar sizes and shapes, they had different particle characteristics and, therefore, had different affinities to the soil used in this study. The isoelectric point of MS2 (3.9) was far lower than those of PhiX174 (6.6) and Qβ (5.3). Therefore, MS2 was more negatively charged than PhiX174 and Qβ in the neutral pH conditions and could attach better to the positively charged sites (aluminum and ferric (hydr)oxides) on the soil particles. It should be noted that the experimental pH conditions of kaolinite-amended soils in this study were between 6.6 and 7.2.
It was reported in the literature that MS2 and PhiX174 have different affinity to adsorbents and porous materials. Park et al. [40] reported that the removal of MS2 in soils amended with steel slag was greater than the removal of PhiX174. MS2 was more negatively charged than PhiX174 at the experimental pH values of 6 to 8. Therefore, MS2 could be removed better than PhiX174, because of stronger electrostatic interactions with positively charged iron oxides present in the steel slag. Kim et al. [41] showed that the removal of MS2 was greater than the removal of PhiX174 by the LDH particles immobilized on the surfaces of quartz sand. MS2 was electrostatically more attractive than PhiX174 to the LDH particles at the experimental pH value of 8.0. Attinti et al. [42] demonstrated that MS2 could be removed more than PhiX174 in positively charged goethite and aluminum oxide-coated sands. Zhang and Jin [43] showed that more MS2 than PhiX174 was removed by positively charged aluminum oxide coated sand. Dowd et al. [44] reported that MS2 adsorption to sandy aquifer materials was 99.4%, which was greater than the adsorption of PhiX174 (85.0%) and Qβ (97.0%) based on their continuously recirculating column experiments.
4. Conclusions
The removals of the bacteriophages MS2, PhiX174, and Qβ in soils amended with kaolinite were investigated. Batch experiments showed that kaolinite was far more effective in the removal of MS2 than montmorillonite and bentonite. In the experiments to study the removal of MS2 in kaolinite-amended (kaolinite content: 0%, 3%, 5%, 7%, and 10%) soils at three different STS ratios (1:10, 1:2, and 1:1), the log removal of MS2 increased with an increase in kaolinite content and STS ratio. In addition, the log removals of PhiX174 and Qβ in kaolinite-amended soils (STS ratio = 1:10) were similar to each other, but far lower than those of MS2 at all of the kaolinite contents (0–10%). In this study, we demonstrated that soil amendment with kaolinite can improve the removal of viruses in soils, but the extent of their removal in kaolinite-amended soils may depend on the types of viruses.