1. Introduction
Wastewater generated from domestic, agricultural, and industrial sources often contains high concentrations of organic and inorganic chemicals, such as hydrocarbon solvents, heavy metals, pesticides, dyes, and phenol-derivative compounds [1]. Phenol and phenol-derivative compounds that are generated from industrial sources, such as refineries, petrochemical, chemical, pharmaceutical, and plastics resin production, are suspected as toxic and carcinogenic compounds [2–4]. Phenol has high stability in the aqueous phase and thus causes serious risk to the aquatic environment. Also, it is detrimental to human health, due to rapid absorption through the skin [5, 6]. Removal methods of phenolic pollutants from aqueous solutions can be divided into three main categories: physical, chemical, and biological treatments. Therefore, several methods, including microbial degradation, adsorption process by activated carbon, chemical oxidation (ozone, hydrogen peroxide, and chlorine dioxide), solvent extraction, and other methods, have been used to remove phenol [7–9]. Each of these methods has some disadvantages in their application. Phenol is toxic, even at low concentrations, and its presence in water can lead further to the formation of substituted compounds during disinfection and oxidation processes. For example, the microbial degradation method is sensitive to temperature, the adsorption technique using activated carbon requires high cost, and chemical oxidation methods such as chlorination and manganese oxide release further toxic compounds in the aquatic environment [10–12]. Thus among them, physical adsorption is generally considered to be the most effective, low-cost and most frequently used method [5].
Recently, many researchers have been using natural material and industrial by-products, including bentonite [13, 14], fly ash [11], and biomass [5] for the removal of inorganic and organic contaminants. Many industrial wastes are regarded as potentially low-cost adsorbents, because they require little processing to increase their sorption capacity. Red mud is a waste residue formed after caustic digestion of bauxite, during the production of alumina [15]. It is a highly alkaline waste material, with pH 10–12.5. The brick red color of the red mud is mainly contributed by iron impurities [16]. It is mainly composed of fine particles, containing aluminum oxide, iron oxide, silica, titanium oxides, and hydroxides [17]. When a ton of alumina is produced, approximately 1 or 2 tons (dry weight) of red mud residues are generated. Due to the alkaline nature of the RM, this solid waste causes a significant impact on the environment. And where alumina industries are located, proper disposal of the waste red mud is a highly necessary task [6, 15, 18–20]. Many studies have been performed to find some practical applications of red mud, such as an additive pigment for mortar and concrete and surface treatment for carbon steel. In recent years, investigations have also been extended to develop red mud as an adsorbent to remove arsenate and toxic heavy metals [21].
In this work, the applicability of red mud for the removal of phenol from aqueous solution was investigated using a batch reactor. The effect of various factors, such as contact time, pH, initial phenol concentrations, and adsorbent dosage, on the removal efficiency of phenol by red mud was also studied.
2. Materials and Methods
All chemicals were analytical grade, and solutions were prepared with deionized water (18 MΩ-cm), from a Hydro-Service reverse osmosis/ion exchange apparatus. Stock solution (1,000 mg/L) of phenol was prepared by dissolving 1 g of phenol in 1 L of deionized water. It was stored in darkness to avoid contact with ambient light. The initial solution pH was adjusted by adding 0.1 M NaOH and HNO3. All experiments were carried out at room temperature (25ºC ± 2ºC).
2.1. Preparation of Activated Red Mud
Red mud obtained from a bauxite mill in Mashhad city, Iran, was washed with deionized water and dried in an oven at 103ºC for 24 hr. The red mud was soaked in 1 N HNO3 at a 1:2 ratio of red mud and nitric acid (w/v) for 24 hr [16]. The obtained material was dried in an oven for 4 hr at 150ºC, and sieved below 100 mesh size. Compared to the preparation process of activated carbon, the activation process of activated red mud is generally performed at a relatively much lower temperature requiring low energy cost.
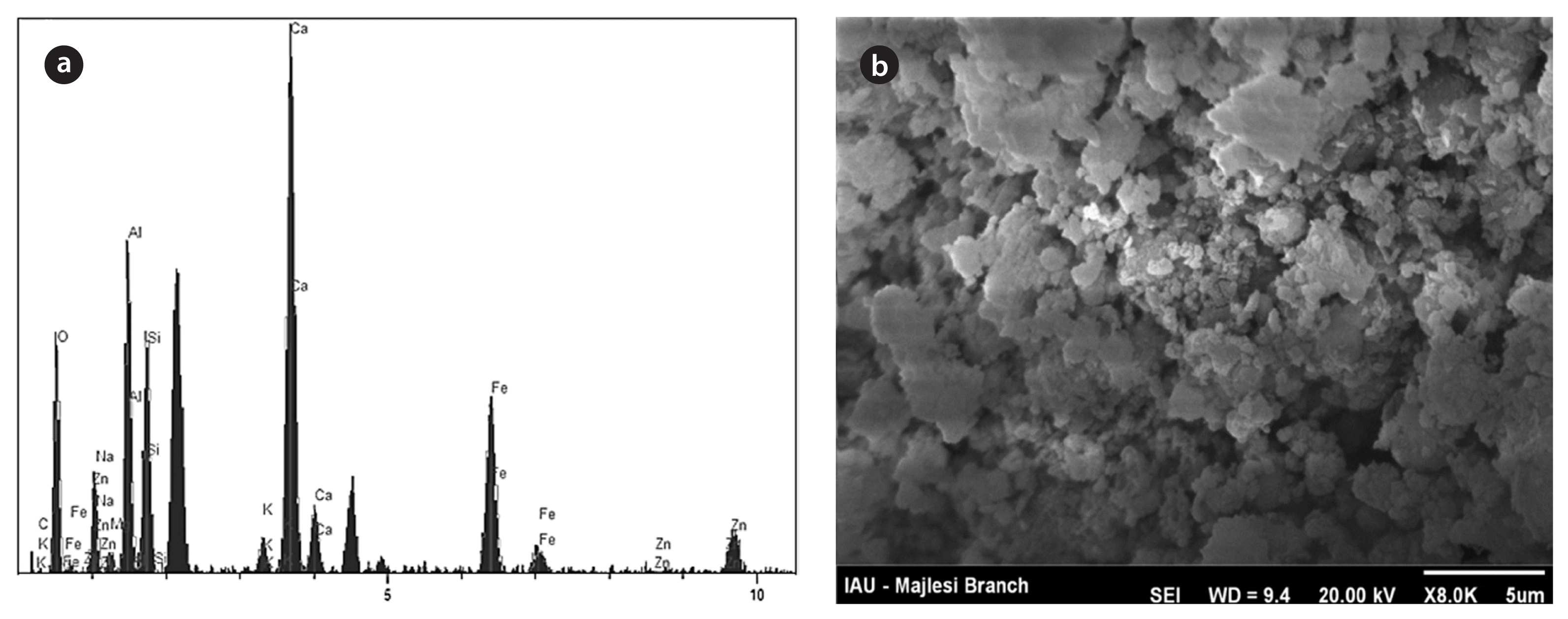
The specific surface area of the activated red mud was determined as 32 m2/g by the Research Institute of Petroleum Industry. The pH of the zero point charge (pHzpc) was determined using a titration method. Briefly, 1.0 g of the activated red mud was suspended in 500 mL of a solution with a constant ionic strength (0.01 M NaCl). The suspension was then titrated with 0.1 M HNO3, and the pH was recorded after stabilization. After titration with HNO3, 0.1 M NaOH was used to bring the pH to 10, and the pH was measured. The microstructure and composition of the activated red mud was studied by using a scanning electron microscope (SEM; AIS-2100, Seron, Uiwang, Korea) and energy dispersive X-ray spectroscopy (EDX; Model 525, 15 kV; Philips, Eindhoven, The Netherlands), respectively. Fig. 1 shows typical EDX patterns and a SEM image of activated red mud. The size of activated red mud was 5 μm on average. Table 1 shows the major compositions of activated red mud used in this work. It is noted that iron and calcium are the major components.
2.2. Adsorption Experiments
The equilibrium time (60 min) was determined from kinetic data obtained at pH 7 with 6 g/L adsorbent and three different initial phenol concentrations(40, 60, and 80 mg/L). In each adsorption experiment, 0.6 g of activated red mud was added into 100 mL of phenol solution of previously adjusted solution pH in 250 mL Erlenmeyer flasks, and the slurry was mixed by rotary mixer (H1–190M)at 160 rpm for 60 min. The slurry samples were centrifuged at 4,000 rpm for 10 min to remove adsorbent, and then residual phenol concentration in aqueous solutions was analyzed by spectrophotometer (Shimadzu UV-160A; Shimadzu, Kyoto, Japan) at a wavelength of 500 nm [22]. Adsorption experiments were conducted with variation of the adsorbent amount (2 to 10 g/L), initial phenol concentration (40 to 80 mg/L), and initial solution pH (3 to 11).
3. Results and Discussion
3.1. The Effect of Contact Time on Phenol Removal
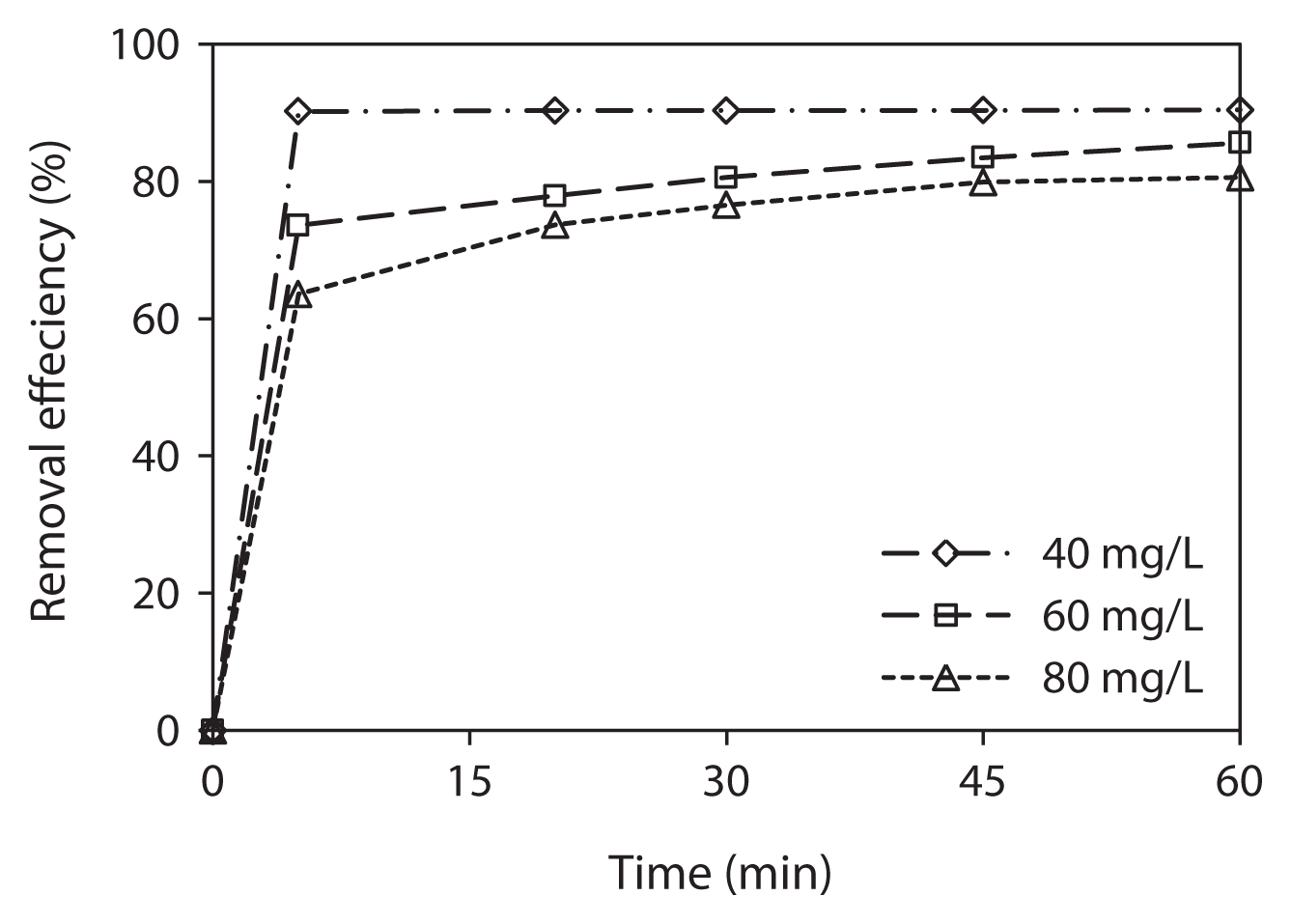
Phenol removal by activated red mud was studied by variation of contact time (5 to 60 min) at different initial phenol concentrations (40 to 80 mg/L), at constant adsorbent dosage (6 g/L), and at solution pH 7. The relationship between contact time and initial phenol concentration is shown in Fig. 2. It is evident from Fig. 2 that time is an important parameter for the adsorption of phenol onto the activated red mud. While increasing the phenol concentration from 40 to 80 mg/L, the removal percentage decreased from 90.3% to 76.6% at initial 30 min of contact time. After that, the removal percentage of phenol slowly increased and ranged from 90.4% to 80.6% at three different phenol concentrations after 60 min. Further increase in the contact time has a negligible effect on the rate of phenol adsorption, and 60 min was determined as a near equilibration time. The decrease in the removal percentage of phenol by increasing initial phenol concentration can be explained in that the adsorbent had a limited number of active sites, which can be easily saturated at higher adsorbate concentration [5, 13, 20, 23].
3.2. The Effect of pH on Phenol Removal
The effect of pH on phenol removal by activated red mud was studied by variation of solution pH (3 to 11) at constant phenol concentration (60 mg/L) and adsorbent dosage (6 g/L) for 60 min. The solution pH was adjusted to 3–11 using 1 M HCl or 1 M NaOH. The relationship between the solution pH and percentage removal of phenol is shown in Fig. 3. By increasing the solution pH, the removal efficiency was increased up to pH 7, but was decreased above neutral pH. The removal percentage of phenol at pH 3, 7, and 11 was 64.5%, 85.6%, and 74.6%, respectively. This phenomenon can be explained by the amphoteric properties of adsorbent and different speciation of phenol molecule with variation of solution pH. The pHzpc of red mud determined from the titration method was 8.96. It was quite similar to the pHzpc(8.5) of red mud reported by other researchers [16, 24].
3.3. Effect of the Adsorbent Dose on Phenol Removal
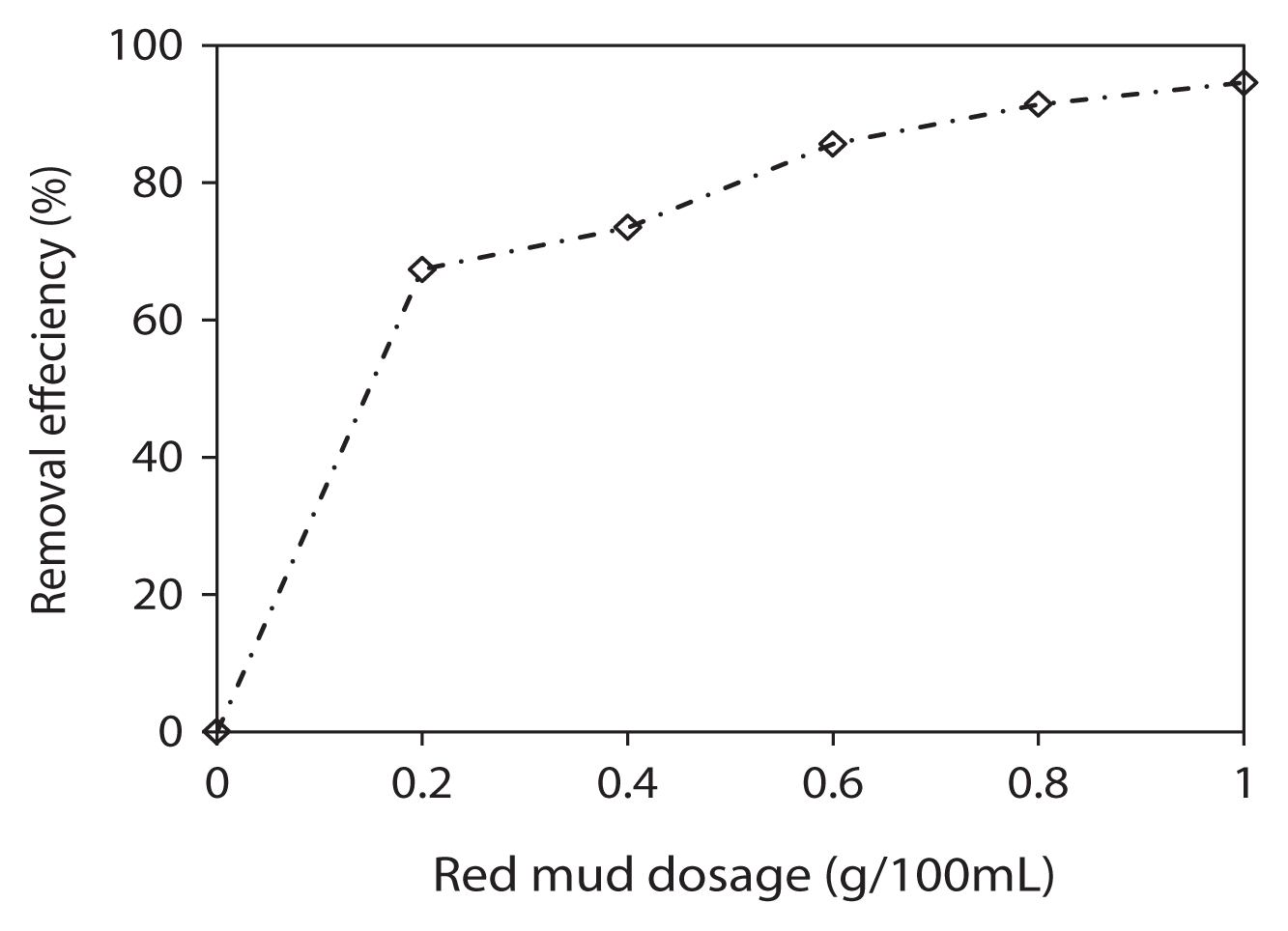
Phenol solution (60 mg/L) was prepared by adding 6 mg of phenol into deionized water in 100 mL volumetric flasks. The pH was adjusted to 7 using 1 M HCl or 1 M NaOH before being transferred into 100 mL Erlenmeyer flasks. The concentration of adsorbent (activated red mud) was varied between 2 g/L and 10 g/L. The mixture was stirred for 60 min at 160 rpm and at room temperature. The relationship between the adsorbent dose and percentage removal of phenol is shown in Fig. 4. The increase in phenol removal with increase in the activated red mud amount is due to the increase of surface area and adsorption sites available for adsorption [5, 15, 25, 26]. Similar observations have also been reported from other research groups [4, 14, 20, 25, 27].
For the modeling of equilibrium data, both Langmuir and Freundlich isotherms were applied [28]. Fig. 5 shows the linear plot of Freundlich and Langmuir isotherms. The value of the correlation coefficient (R2 = 0.99) indicates that there was good agreement between the experimental equilibrium data and the Freundlich isotherm. Isotherm constants for the phenol adsorption are presented in Table 2. Table 2 shows comparison data for the removal capacity of phenol by several adsorbents, such as sawdust, granular activated carbon, bagasse fly ash, bentonite, and neutralized red mud, including activated red mud [14, 20, 29–31]. Although the maximum adsorption capacity (qm) of phenol by the activated red mud obtained by the Langmuir equation was relatively less than that by granular activated carbon and bagasse fly ash, it was greater than that by sawdust and bentonite. The physical properties of commercial granular activated carbon were an apparent density of 0.4 g/cm3, particle size of 1.4 mm, and average pore diameter of 18 Å [30].
3.4. Adsorption Kinetics
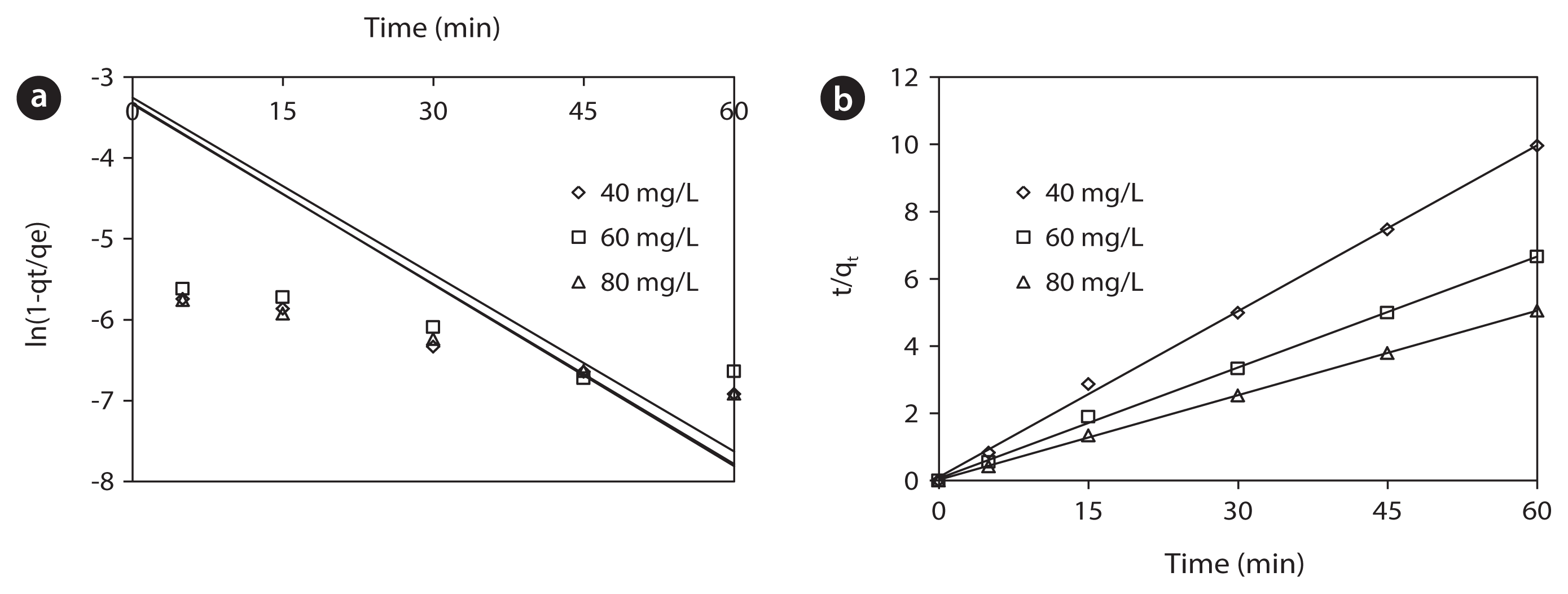
Kinetics studies were conducted in a series of 250 mL Erlenmeyer flasks, filled with 100 mL of phenol (40, 60, and 80 mg/L) and adsorbent (6 g/L), at pH 7±0.2. At specified time interval, the samples were separated and analyzed for their residual phenol concentrations. The kinetics of phenol onto the activated red mud was analyzed by pseudo-first-order and pseudo-second-order models at various initial phenol concentrations. Fig. 6 shows linear plots of pseudo-first-order, and pseudo-second-order models for the removal of phenol onto the activated red mud. The obtained values of k1, k2, and qe are summarized in Table 3. The value of the correlation coefficient (R2 = 0.999) indicates that the adsorption kinetic data of phenol onto the activated red mud is well described with the pseudo-second-order model. A good fit of experimental data to the pseudo-second-order model suggests that the overall rate of adsorption process appears to be controlled by the chemisorption process, which involved forces through sharing or exchange of electrons between the activated red mud and phenol. This result is consistent with the previously reported results [32]
One of the important issues for the application of adsorbents is regeneration of the adsorbent after saturation. There were some reports for the regeneration of activated red mud after saturation and the reuse of it [24, 33, 34]. Considering this result and the removal efficiency obtained by this work, activated red mud can be used for the treatment of aqueous solutions containing phenol as a low cost adsorbent with high efficiency.
4. Conclusions
In this study, activated red mud containing iron and calcium as major components was applied to treat synthetic wastewater in a batch reactor. The removal percentage of phenol was affected by the solution pH and shows maximum removal capacity at neutral pH. The removal percentage of phenol was decreased by increasing the initial phenol concentrations. Adsorption results show that the equilibrium data follow the Freundlich isotherm, and the kinetic data was well described by a pseudo-second-order kinetic model. From this work, activated red mud can be used for the treatment of aqueous solutions containing phenol as a low cost adsorbent with high efficiency. Considering these results, activated red mud shows a promising adsorption capacity for phenol and can be used as a suitable adsorbent for the treatment of wastewater containing phenol.